Citric Acid Promotes Immune Function by Modulating the Intestinal Barrier
- PMID: 38279237
- PMCID: PMC10817003
- DOI: 10.3390/ijms25021239
Citric Acid Promotes Immune Function by Modulating the Intestinal Barrier
Abstract
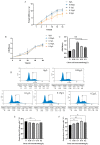
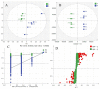
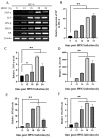
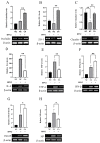
Amidst increasing concern about antibiotic resistance resulting from the overuse of antibiotics, there is a growing interest in exploring alternative agents. One such agent is citric acid, an organic compound commonly used for various applications. Our research findings indicate that the inclusion of citric acid can have several beneficial effects on the tight junctions found in the mouse intestine. Firstly, the study suggests that citric acid may contribute to weight gain by stimulating the growth of intestinal epithelial cells (IE-6). Citric acid enhances the small intestinal villus-crypt ratio in mice, thereby promoting intestinal structural morphology. Additionally, citric acid has been found to increase the population of beneficial intestinal microorganisms, including Bifidobacterium and Lactobacillus. It also promotes the expression of important protein genes such as occludin, ZO-1, and claudin-1, which play crucial roles in maintaining the integrity of the tight junction barrier in the intestines. Furthermore, in infected IEC-6 cells with H9N2 avian influenza virus, citric acid augmented the expression of genes closely associated with the influenza virus infection. Moreover, it reduces the inflammatory response caused by the viral infection and thwarted influenza virus replication. These findings suggest that citric acid fortifies the intestinal tight junction barrier, inhibits the replication of influenza viruses targeting the intestinal tract, and boosts intestinal immune function.
Keywords: H9N2 influenza virus; citric acid; gut microorganisms; intestinal barrier; metabolomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kuldeep D., Ruchi T., Ullah K.R., Sandip C., Marappan G., Kumaragurubaran K., Mani S., Arumugam D.P., Tulasi S.L. Growth Promoters and Novel Feed Additives Improving Poultry Production and Health, Bioactive Principles and Beneficial Applications: The Trends and Advances—A Review. Int. J. Pharmacol. 2014;10:129–159.
-
- Yadav A.S., Kolluri G., Gopi M., Karthik K., Malik Y.S., Dhama K. Exploring alternatives to antibiotics as health promoting agents in poultry—A review. J. Exp. Biol. Agric. Sci. 2016;4:368–383. doi: 10.18006/2016.4(3S).368.383. - DOI
-
- Suzuki C., Hatayama N., Ogawa T., Nanizawa E., Otsuka S., Hata K., Seno H., Naito M., Hirai S. Cardioprotection via Metabolism for Rat Heart Preservation Using the High-Pressure Gaseous Mixture of Carbon Monoxide and Oxygen. Int. J. Mol. Sci. 2020;21:8858. doi: 10.3390/ijms21228858. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

