Discovery of Novel Diphenyl Acrylonitrile Derivatives That Promote Adult Rats' Hippocampal Neurogenesis
- PMID: 38279241
- PMCID: PMC10816640
- DOI: 10.3390/ijms25021241
Discovery of Novel Diphenyl Acrylonitrile Derivatives That Promote Adult Rats' Hippocampal Neurogenesis
Abstract
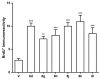
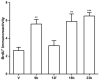
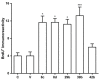
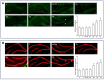
We previously discovered WS-6 as a new antidepressant in correlation to its function of stimulating neurogenesis. Herein, several different scaffolds (stilbene, 1,3-diphenyl 1-propene, 1,3-diphenyl 2-propene, 1,2-diphenyl acrylo-1-nitrile, 1,2-diphenyl acrylo-2-nitrile, 1,3-diphenyl trimethylamine), further varied through substitutions of twelve amide substituents plus the addition of a methylene unit and an inverted amide, were examined to elucidate the SARs for promoting adult rat neurogenesis. Most of the compounds could stimulate proliferation of progenitors, but just a few chemicals possessing a specific structural profile, exemplified by diphenyl acrylonitrile 29b, 32a, and 32b, showed better activity than the clinical drug NSI-189 in promoting newborn cells differentiation into mature neurons. The most potent diphenyl acrylonitrile 32b had an excellent brain AUC to plasma AUC ratio (B/P = 1.6), suggesting its potential for further development as a new lead.
Keywords: SARs; differentiation; diphenyl acrylonitrile; neurogenesis; proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
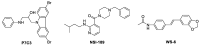




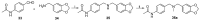
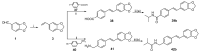



References
-
- Moreno-Jimenez E.P., Flor-Garcia M., Terreros-Roncal J., Rabano A., Cafini F., Pallas-Bazarra N., Avila J., Llorens-Martin M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019;25:554–564. doi: 10.1038/s41591-019-0375-9. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

