WNT/β-catenin regulatory roles on PD-(L)1 and immunotherapy responses
- PMID: 38280119
- PMCID: PMC10822012
- DOI: 10.1007/s10238-023-01274-z
WNT/β-catenin regulatory roles on PD-(L)1 and immunotherapy responses
Abstract
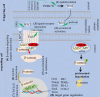
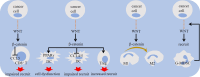
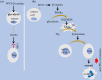
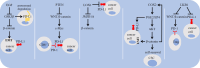
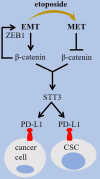
Dysregulation of WNT/β-catenin is a hallmark of many cancer types and a key mediator of metastasis in solid tumors. Overactive β-catenin signaling hampers dendritic cell (DC) recruitment, promotes CD8+ T cell exclusion and increases the population of regulatory T cells (Tregs). The activity of WNT/β-catenin also induces the expression of programmed death-ligand 1 (PD-L1) on tumor cells and promotes programmed death-1 (PD-1) upregulation. Increased activity of WNT/β-catenin signaling after anti-PD-1 therapy is indicative of a possible implication of this signaling in bypassing immune checkpoint inhibitor (ICI) therapy. This review is aimed at giving a comprehensive overview of the WNT/β-catenin regulatory roles on PD-1/PD-L1 axis in tumor immune ecosystem, discussing about key mechanistic events contributed to the WNT/β-catenin-mediated bypass of ICI therapy, and representing inhibitors of this signaling as promising combinatory regimen to go with anti-PD-(L)1 in cancer immunotherapy. Ideas presented in this review imply the synergistic efficacy of such combination therapy in rendering durable anti-tumor immunity.
Keywords: Immune checkpoint inhibitor (ICI); Programmed death-1 (PD-1); Programmed death-ligand 1 (PD-L1); Resistance; Tumor microenvironment (TME); β-catenin.
© 2024. The Author(s).
Conflict of interest statement
None to declare.
Figures
References
-
- Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

