Long-Term Survival and Stable Disease in a Patient with Extensive-Stage Small-Cell Lung Cancer after Treatment with Carboplatin, Etoposide and Atezolizumab
- PMID: 38280181
- PMCID: PMC10881916
- DOI: 10.1007/s40487-023-00257-0
Long-Term Survival and Stable Disease in a Patient with Extensive-Stage Small-Cell Lung Cancer after Treatment with Carboplatin, Etoposide and Atezolizumab
Abstract
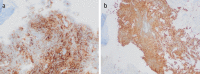
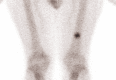
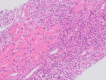
Survival beyond 2 years is rare in patients with extensive-stage small-cell lung cancer (ES-SCLC) treated with chemotherapy alone. We describe a patient with ES-SCLC who was treated with carboplatin, etoposide and the programmed death-ligand 1 inhibitor atezolizumab in the IMpower133 study (ClinicalTrials.gov registration: NCT02763579) and who achieved exceptionally long-term survival. Treatment-naïve patients with ES-SCLC (n = 403) were included in the IMpower133 study, and the identified patient had been randomised to the investigational treatment arm, where patients received induction therapy with carboplatin and etoposide plus atezolizumab for four 21-day cycles, followed by ongoing maintenance therapy with atezolizumab. The patient had achieved a partial response after induction therapy, and then received seven cycles of atezolizumab maintenance therapy until immune-related toxicities necessitated discontinuation. The patient was alive with an ongoing response and excellent performance status more than 6 years after starting treatment and 5 years after discontinuing atezolizumab maintenance. In conclusion, this patient with ES-SCLC from the IMpower133 study is a rare example of ongoing survival more than 6 years beyond diagnosis and the start of treatment with first-line atezolizumab. This demonstrates the potential durability of response with immunotherapy.
Keywords: Atezolizumab; Carboplatin; Etoposide; Long-term survival; Small-cell lung cancer.
© 2024. The Author(s).
Conflict of interest statement
Stephen V. Liu has been on an advisory board for or acted as a consultant for AbbVie, Amgen, AstraZeneca, Blueprint, Boehringer Ingelheim, Bristol-Myers Squibb, Catalyst, Daiichi Sankyo, Eisai, Elevation Oncology, Genentech/Roche, Gilead, Guardant Health, Janssen, Jazz Pharmaceuticals, Merck/MSD, Merus, Novartis, Regeneron, Sanofi, Takeda and Turning Point Therapeutics and has received research funding (to institution) from Alkermes, Elevation Oncology, Genentech, Gilead, Merck, Nuvalent, RAPT and Turning Point Therapeutics. Reyes Bernabé, Amparo Sánchez-Gastaldo and Miriam Alonso García declare that they have no competing interests.
Figures

Similar articles
-
Clinical and molecular characterization of long-term survivors with extensive-stage small cell lung cancer treated with first-line atezolizumab plus carboplatin and etoposide.Lung Cancer. 2023 Dec;186:107418. doi: 10.1016/j.lungcan.2023.107418. Epub 2023 Oct 31. Lung Cancer. 2023. PMID: 37931445 Clinical Trial.
-
Brief Report: Exploratory Analysis of Maintenance Therapy in Patients With Extensive-Stage SCLC Treated First Line With Atezolizumab Plus Carboplatin and Etoposide.J Thorac Oncol. 2022 Sep;17(9):1122-1129. doi: 10.1016/j.jtho.2022.05.016. Epub 2022 Jun 25. J Thorac Oncol. 2022. PMID: 35764236 Clinical Trial.
-
Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): a randomized phase I/III trial.Ann Oncol. 2020 Feb;31(2):310-317. doi: 10.1016/j.annonc.2019.10.021. Epub 2019 Dec 9. Ann Oncol. 2020. PMID: 31959349 Clinical Trial.
-
Atezolizumab: A Review in Extensive-Stage SCLC.Drugs. 2020 Oct;80(15):1587-1594. doi: 10.1007/s40265-020-01398-6. Drugs. 2020. PMID: 32990939 Review.
-
FDA Approval Summary: Atezolizumab and Durvalumab in Combination with Platinum-Based Chemotherapy in Extensive Stage Small Cell Lung Cancer.Oncologist. 2021 May;26(5):433-438. doi: 10.1002/onco.13752. Epub 2021 Mar 25. Oncologist. 2021. PMID: 33687763 Free PMC article. Review.
Cited by
-
Durable Response to Atezolizumab in Extensive-Stage Small-Cell Lung Cancer Leading to 60 Months Overall Survival: A Case Report.Curr Oncol. 2024 Jun 27;31(7):3682-3689. doi: 10.3390/curroncol31070271. Curr Oncol. 2024. PMID: 39057143 Free PMC article.
References
-
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. - DOI - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in oncology: small cell lung cancer. Version 2.2022. 2021. https://www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed 10 Feb 2022. - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

