Long-Term Survival and Stable Disease in a Patient with Extensive-Stage Small-Cell Lung Cancer after Treatment with Carboplatin, Etoposide and Atezolizumab
- PMID: 38280181
- PMCID: PMC10881916
- DOI: 10.1007/s40487-023-00257-0
Long-Term Survival and Stable Disease in a Patient with Extensive-Stage Small-Cell Lung Cancer after Treatment with Carboplatin, Etoposide and Atezolizumab
Abstract
Survival beyond 2 years is rare in patients with extensive-stage small-cell lung cancer (ES-SCLC) treated with chemotherapy alone. We describe a patient with ES-SCLC who was treated with carboplatin, etoposide and the programmed death-ligand 1 inhibitor atezolizumab in the IMpower133 study (ClinicalTrials.gov registration: NCT02763579) and who achieved exceptionally long-term survival. Treatment-naïve patients with ES-SCLC (n = 403) were included in the IMpower133 study, and the identified patient had been randomised to the investigational treatment arm, where patients received induction therapy with carboplatin and etoposide plus atezolizumab for four 21-day cycles, followed by ongoing maintenance therapy with atezolizumab. The patient had achieved a partial response after induction therapy, and then received seven cycles of atezolizumab maintenance therapy until immune-related toxicities necessitated discontinuation. The patient was alive with an ongoing response and excellent performance status more than 6 years after starting treatment and 5 years after discontinuing atezolizumab maintenance. In conclusion, this patient with ES-SCLC from the IMpower133 study is a rare example of ongoing survival more than 6 years beyond diagnosis and the start of treatment with first-line atezolizumab. This demonstrates the potential durability of response with immunotherapy.
Keywords: Atezolizumab; Carboplatin; Etoposide; Long-term survival; Small-cell lung cancer.
© 2024. The Author(s).
Conflict of interest statement
Stephen V. Liu has been on an advisory board for or acted as a consultant for AbbVie, Amgen, AstraZeneca, Blueprint, Boehringer Ingelheim, Bristol-Myers Squibb, Catalyst, Daiichi Sankyo, Eisai, Elevation Oncology, Genentech/Roche, Gilead, Guardant Health, Janssen, Jazz Pharmaceuticals, Merck/MSD, Merus, Novartis, Regeneron, Sanofi, Takeda and Turning Point Therapeutics and has received research funding (to institution) from Alkermes, Elevation Oncology, Genentech, Gilead, Merck, Nuvalent, RAPT and Turning Point Therapeutics. Reyes Bernabé, Amparo Sánchez-Gastaldo and Miriam Alonso García declare that they have no competing interests.
Figures

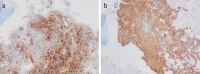
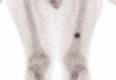
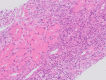
References
-
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. - DOI - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in oncology: small cell lung cancer. Version 2.2022. 2021. https://www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed 10 Feb 2022. - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

