Treatment Patterns, Healthcare Resource Utilization, and Costs of Patients With Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma in the US
- PMID: 38280190
- PMCID: PMC10911928
- DOI: 10.1093/oncolo/oyad324
Treatment Patterns, Healthcare Resource Utilization, and Costs of Patients With Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma in the US
Abstract
Background: Chronic lymphocytic leukemia (CLL) is the most common type of leukemia among US adults and has experienced a rapidly evolving treatment landscape; yet current data on treatment patterns in clinical practice and economic burden are limited. This study aimed to provide an up-to-date description of real-world characteristics, treatments, and costs of patients with CLL or small lymphocytic lymphoma (SLL).
Materials and methods: Using retrospective data from the Optum Clinformatics DataMart database (January 2013 to December 2021), adults with diagnosis codes for CLL/SLL on two different dates were selected. An adapted algorithm identified lines of therapy (LOT). Treatment patterns were stratified by the index year pre- and post-2018. Healthcare resource utilization and costs were evaluated per patient-years.
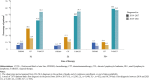
Results: A total of 18 418 patients with CLL/SLL were identified, 5226 patients (28%) were treated with ≥1 LOT and 1728 (9%) with ≥2 LOT. Among patients diagnosed with CLL in 2014-2017 and ≥1 LOT (N = 2585), 42% used targeted therapy and 30% used chemoimmunotherapy in first line (1L). The corresponding proportions of patients diagnosed with CLL in 2018-2021 (N = 2641) were 54% and 16%, respectively. Total costs were numerically 3.5 times higher and 4.9 times higher compared with baseline costs among patients treated with 1L+ and 3L+, respectively.
Conclusion: This study documented the real-world change in CLL treatment landscape and the substantial economic burden of patients with CLL/SLL. Specifically, targeted therapies were increasingly used as 1L treatments and they were part of more than half of 1L regimens in recent years (2018-2021).
Keywords: chronic lymphocytic leukemia; economic burden; healthcare administrative claims; treatment.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
Enrico Zanardo, Dominique Lejeune, and François Laliberté are employees of Analysis Group, Inc., a consulting company that has received research funds from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, to conduct this study. Xiaoqin Yang, Enrico De Nigris, Eric Sarpong, and Mohammed Farooqui are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

