Selective C-H Halogenation of Alkenes and Alkynes Using Flavin-Dependent Halogenases
- PMID: 38280216
- PMCID: PMC10947852
- DOI: 10.1002/anie.202317860
Selective C-H Halogenation of Alkenes and Alkynes Using Flavin-Dependent Halogenases
Abstract
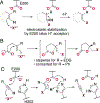
Single component flavin-dependent halogenases (FDHs) possess both flavin reductase and FDH activity in a single enzyme. We recently reported that the single component FDH AetF catalyzes site-selective bromination and iodination of a variety of aromatic substrates and enantioselective bromolactonization and iodoetherification of styrenes bearing pendant carboxylic acid or alcohol substituents. Given this inherent reactivity and selectivity, we explored the utility of AetF as catalyst for alkene and alkyne C-H halogenation. We find that AetF catalyzes halogenation of a range of 1,1-disubstituted styrenes, often with high stereoselectivity. Despite the utility of haloalkenes for cross-coupling and other applications, accessing these compounds in a stereoselective manner typically requires functional group interconversion processes, and selective halogenation of 1,1'-disubstituted olefins remains rare. We also establish that AetF and homologues of this enzyme can halogenate terminal alkynes. Mutagenesis studies and deuterium kinetic isotope effects are used to support a mechanistic proposal involving covalent catalysis for halogenation of unactivated alkynes by AetF homologues. These findings expand the scope of FDH catalysis and continue to show the unique utility of single component FDHs for biocatalysis.
© 2024 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Figures
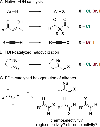


Similar articles
-
Identifying and Engineering Flavin Dependent Halogenases for Selective Biocatalysis.Acc Chem Res. 2024 Aug 6;57(15):2067-2079. doi: 10.1021/acs.accounts.4c00172. Epub 2024 Jul 22. Acc Chem Res. 2024. PMID: 39038085 Free PMC article.
-
The Single-Component Flavin Reductase/Flavin-Dependent Halogenase AetF is a Versatile Catalyst for Selective Bromination and Iodination of Arenes and Olefins.Angew Chem Int Ed Engl. 2022 Dec 19;61(51):e202214610. doi: 10.1002/anie.202214610. Epub 2022 Nov 22. Angew Chem Int Ed Engl. 2022. PMID: 36282507 Free PMC article.
-
Asymmetric catalysis by flavin-dependent halogenases.Chirality. 2023 Aug;35(8):452-460. doi: 10.1002/chir.23550. Epub 2023 Mar 14. Chirality. 2023. PMID: 36916449 Free PMC article. Review.
-
Flavin-dependent halogenases catalyze enantioselective olefin halocyclization.Nat Commun. 2021 Jun 1;12(1):3268. doi: 10.1038/s41467-021-23503-3. Nat Commun. 2021. PMID: 34075034 Free PMC article.
-
Understanding and Improving the Activity of Flavin-Dependent Halogenases via Random and Targeted Mutagenesis.Annu Rev Biochem. 2018 Jun 20;87:159-185. doi: 10.1146/annurev-biochem-062917-012042. Epub 2018 Mar 28. Annu Rev Biochem. 2018. PMID: 29589959 Free PMC article. Review.
Cited by
-
Unlocking the catalytic precision of ligand-controlled enzymatic halogenation.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2409479122. doi: 10.1073/pnas.2409479122. Epub 2024 Dec 30. Proc Natl Acad Sci U S A. 2025. PMID: 39793036 Free PMC article.
-
Ring-Opening Chlorination and Bromination of Pyrazolopyridines and Isoxazoles.Chemistry. 2025 Jul 22;31(41):e202501589. doi: 10.1002/chem.202501589. Epub 2025 Jul 4. Chemistry. 2025. PMID: 40613443 Free PMC article.
-
Exploiting Archaeal/Thermostable Enzymes in Synthetic Chemistry: Back to the Future?ChemCatChem. 2024 Nov 11;16(21):e202400835. doi: 10.1002/cctc.202400835. Epub 2024 Jul 8. ChemCatChem. 2024. PMID: 40417414
-
Giant polyketide synthase enzymes biosynthesize a giant marine polyether biotoxin.bioRxiv [Preprint]. 2024 Jan 31:2024.01.29.577497. doi: 10.1101/2024.01.29.577497. bioRxiv. 2024. Update in: Science. 2024 Aug 9;385(6709):671-678. doi: 10.1126/science.ado3290. PMID: 38352448 Free PMC article. Updated. Preprint.
-
Metagenomic Identification of Brominated Indole Biosynthetic Machinery from Cyanobacteria.J Nat Prod. 2025 Jul 25;88(7):1734-1742. doi: 10.1021/acs.jnatprod.5c00492. Epub 2025 Jul 10. J Nat Prod. 2025. PMID: 40637245 Free PMC article.
References
-
- Hernandes M, Cavalcanti S, Moreira D, de A W Junior, Leite A, Curr. Drug Targets 2010, 11, 303–314. - PubMed
-
- Jeschke P, Pest Man. Sci. 2010, 66, 10–27. - PubMed
-
- Podgoršek A, Zupan M, Iskra J, Angew. Chem. Int. Ed. 2009, 48, 8424–8450. - PubMed
-
- Smith K, El-Hiti G, Current Organic Synthesis 2004, 1, 253–274.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

