Mitochondrial protein synthesis quality control
- PMID: 38280230
- PMCID: PMC11112378
- DOI: 10.1093/hmg/ddae012
Mitochondrial protein synthesis quality control
Abstract
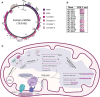
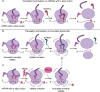
Human mitochondrial DNA is one of the most simplified cellular genomes and facilitates compartmentalized gene expression. Within the organelle, there is no physical barrier to separate transcription and translation, nor is there evidence that quality control surveillance pathways are active to prevent translation on faulty mRNA transcripts. Mitochondrial ribosomes synthesize 13 hydrophobic proteins that require co-translational insertion into the inner membrane of the organelle. To maintain the integrity of the inner membrane, which is essential for organelle function, requires responsive quality control mechanisms to recognize aberrations in protein synthesis. In this review, we explore how defects in mitochondrial protein synthesis can arise due to the culmination of inherent mistakes that occur throughout the steps of gene expression. In turn, we examine the stepwise series of quality control processes that are needed to eliminate any mistakes that would perturb organelle homeostasis. We aim to provide an integrated view on the quality control mechanisms of mitochondrial protein synthesis and to identify promising avenues for future research.
Keywords: AFG3L2; MTRFR; OMA1; OPA1; OXA1L; RNA processing; cell stress; co-translational quality control; fusion open reading frames; membrane morphology; mitochondria; non-stop mRNA; post-transcriptional; protein synthesis; proteostasis; ribosome quality control; ribosomes.
© The Author(s) 2024. Published by Oxford University Press.
Figures

Similar articles
-
Mitochondrial stress response triggered by defects in protein synthesis quality control.Life Sci Alliance. 2019 Jan 25;2(1):e201800219. doi: 10.26508/lsa.201800219. Print 2019 Feb. Life Sci Alliance. 2019. PMID: 30683687 Free PMC article.
-
Sucrose Gradient Analysis of Human Mitochondrial Ribosomes and RNA.Methods Mol Biol. 2023;2661:101-117. doi: 10.1007/978-1-0716-3171-3_7. Methods Mol Biol. 2023. PMID: 37166634
-
Structure and Function of the Mitochondrial Ribosome.Annu Rev Biochem. 2016 Jun 2;85:103-32. doi: 10.1146/annurev-biochem-060815-014343. Epub 2016 Mar 24. Annu Rev Biochem. 2016. PMID: 27023846 Review.
-
Organization and Regulation of Mitochondrial Protein Synthesis.Annu Rev Biochem. 2016 Jun 2;85:77-101. doi: 10.1146/annurev-biochem-060815-014334. Epub 2016 Jan 18. Annu Rev Biochem. 2016. PMID: 26789594 Review.
-
Mitochondrial Translation Efficiency Controls Cytoplasmic Protein Homeostasis.Cell Metab. 2018 Jun 5;27(6):1309-1322.e6. doi: 10.1016/j.cmet.2018.04.011. Epub 2018 May 10. Cell Metab. 2018. PMID: 29754951
Cited by
-
Complete Mitogenome sequencing of the fish louse Argulus japonicus (Crustacea: Branchiura): Comparative analyses and phylogenetic implications.Front Vet Sci. 2024 Mar 25;11:1376898. doi: 10.3389/fvets.2024.1376898. eCollection 2024. Front Vet Sci. 2024. PMID: 38590542 Free PMC article.
-
Important Role of Mitochondrial Dysfunction in Immune Triggering and Inflammatory Response in Rheumatoid Arthritis.J Inflamm Res. 2024 Dec 27;17:11631-11657. doi: 10.2147/JIR.S499473. eCollection 2024. J Inflamm Res. 2024. PMID: 39741752 Free PMC article. Review.
-
Control of Mitochondrial Quality: A Promising Target for Diabetic Kidney Disease Treatment.Kidney Int Rep. 2024 Dec 31;10(4):994-1010. doi: 10.1016/j.ekir.2024.12.029. eCollection 2025 Apr. Kidney Int Rep. 2024. PMID: 40303215 Free PMC article. Review.
References
-
- Suomalainen A, Battersby BJ. Mitochondrial diseases: the contribution of organelle stress responses to pathology. Nat Rev Mol Cell Biol 2018;19:77–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

