Immunomodulatory effects and improved outcomes with cisplatin- versus carboplatin-based chemotherapy plus atezolizumab in urothelial cancer
- PMID: 38280376
- PMCID: PMC10897541
- DOI: 10.1016/j.xcrm.2024.101393
Immunomodulatory effects and improved outcomes with cisplatin- versus carboplatin-based chemotherapy plus atezolizumab in urothelial cancer
Abstract
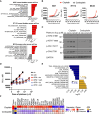
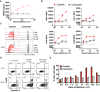
In metastatic urothelial cancer (mUC), cisplatin versus carboplatin leads to durable disease control in a subset of patients. The IMvigor130 trial reveals more favorable effects with atezolizumab combined with gemcitabine and cisplatin (GemCis) versus gemcitabine and carboplatin (GemCarbo). This study investigates the immunomodulatory effects of cisplatin as a potential explanation for these observations. Our findings indicate that improved outcomes with GemCis versus GemCarbo are primarily observed in patients with pretreatment tumors exhibiting features of restrained adaptive immunity. In addition, GemCis versus GemCarbo ± atezolizumab induces transcriptional changes in circulating immune cells, including upregulation of antigen presentation and T cell activation programs. In vitro experiments demonstrate that cisplatin, compared with carboplatin, exerts direct immunomodulatory effects on cancer cells, promoting dendritic cell activation and antigen-specific T cell killing. These results underscore the key role of immune modulation in cisplatin's efficacy in mUC and highlight the importance of specific chemotherapy backbones in immunotherapy combination regimens.
Trial registration: ClinicalTrials.gov NCT02807636.
Keywords: PD-1 blockade; PD-L1 blockade; bladder cancer; carboplatin; chemotherapy; cisplatin; immune checkpoint blockade; immunogenic cell death; single cell RNA sequencing; urothelial cancer.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.D.G. has received grants or contracts from Bristol Myers Squibb, Novartis, Dendreon, AstraZeneca, and Merck; and has received consulting fees from Bristol Myers Squibb, Merck, Genentech Inc., AstraZeneca, Pfizer, EMD Serono, Seagen, Janssen, Numab, Dragonfly, GlaxoSmithKline, Basilea, UroGen, RapptaTherapeutics, Alligator, Silverback, Fujifilm, and Curis. X.G., D.R., A.S.R., H.M.S., R.B., K.Y., E.V., R.H., C.-J.H., Y.L., D.V., P.W., I.M., S.S., R.J., and S.M. are employees of Genentech Inc., and hold stocks or stock options in Roche/Genentech Inc. H.L. is an employee of Hoffmann-La Roche Ltd, Canada. L.W., J.Z., and H.Y. are employees of Sema4. E.H. was employed at Immunai during study conduct. R.H.H. and E. Kiner are employees of Immunai. A.B. has served as a consultant or advisor to Roche, Bristol Myers Squibb, MSD, and Pfizer; has received honoraria from Bristol Myers Squibb, MSD, and Pfizer; and has received educational and research grants from Pfizer, Pierre-Fabre, and Bristol Myers Squibb. M.D.S. has received consulting fees from AAA, Accord, Amgen, Astellas, AstraZeneca, Basilea, Bayer, Bioclin, Bristol Myers Squibb, Eisai, Ferring, Immunomedics, Ipsen, Janssen, MSD, Merck, Novartis, Pfizer, Pierre Fabre Oncology, Roche, Sandoz, Sanofi, and Seagen; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AAA, Accord, Amgen, Astellas, AstraZeneca, Basilea, Bayer, Bioclin, Bristol Myers Squibb, Eisai, Ferring, Immunomedics, Ipsen, Janssen, MSD, Merck, Novartis, Pfizer, Pierre Fabre Oncology, Roche, Sandoz, Sanofi, and Seagen; has received support for attending meetings and/or travel from AAA, Accord, Amgen, Astellas, AstraZeneca, Basilea, Bayer, Bioclin, Bristol Myers Squibb, Eisai, Ferring, Immunomedics, Ipsen, Janssen, MSD, Merck, Novartis, Pfizer, Pierre Fabre Oncology, Roche, Sandoz, Sanofi, and Seagen; has participated in a Data Safety Monitoring Board or Advisory Board for Roche, Orion, and CR-UK; and has other financial or non-financial interests by having worked on the ESMO guidelines on bladder cancer and the German S3 Leitlinie Blasenkarzinom. I.D.D. has participated in a Data Safety Monitoring Board or Advisory Board for Roche/Genentech Inc. (WO30070 [IMvigor130] international steering committee; unpaid) and Merck/Pfizer (APAC GU Advisory Board; unpaid: honoraria are invoiced by and paid directly to ANZUP Cancer Trials Group); and has a leadership or fiduciary role in the ANZUP Cancer Trials Group (as a Director and Board Chair; unpaid). J.A.A. has received third-party service as collaboration in the selection, and management of administrative requirements for the participation of SOGUG centers in the study from SOGUG during the conduct of the study; speaking or consulting fees from Bristol Myers Squibb, MSD, Roche, Astellas, Janssen Cilag, Pfizer, Novartis, and Bayer outside the submitted work; travel support from Bristol Myers Squibb, MSD, Roche, and Janssen Cilag outside the submitted work; research funding (from SOGUG) outside the submitted work from Bristol Myers Squibb, Novartis, and Pierre Fabre; and has participated in industry-sponsored clinical trials for Bristol Myers Squibb, MSD, Roche, Astellas, Janssen Cilag, Pfizer, and Novartis outside the submitted work. E. Kikuchi has received grants or contracts (through his institution) from Takeda, Nippon Kayaku, and Taiho; has received consulting fees from Chugai, Nippon Kayaku, MSD, Takeda, Pfizer, Astellas, Bristol Myers Squibb, and Yansen; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Takeda, Nippon Kayaku, and Taiho; and has received consulting fees from Chugai, Nippon Kayaku, MSD, Takeda, Taiho, AstraZeneca, Pfizer, Astellas, Bristol Myers Squibb, and Yansen. S.B. is an employee of Roche Products Ltd, UK, holds stocks or stock options in Roche, and has other financial or non-financial interests in Roche. C.L. is an employee of Roche Products Ltd, UK, holds stocks or stock options in Roche, and has other financial or non-financial interests in Roche. P.C.B. has participated in advisory boards for Janssen, Merck, Roche/Genentech Inc., Bristol Myers Squibb, Urogen, EMD Serono, Bayer, Astellas, AbbVie, AstraZeneca, Ferring, H3-Biomedicine, Sanofi, Pfizer, Prokarium, Protara Therapeutics, Stimit, and Verity; has received payment or honoraria as a speaker from Janssen, Bayer, Ferring, H3-Biomedicine, and Pfizer; has participated in clinical trials for Roche/Genentech Inc., Bristol Myers Squibb, and AstraZeneca; and has received non-financial support (scientific collaboration on bladder cancer genomics/transcriptomics) from Decipher Biosciences/Veracyte. E.G. has received grants or contracts from Astellas, AstraZeneca, Ipsen, Merck KGaA, and Pfizer; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Esteve, Ipsen, Janssen, Lilly, Merck KGaA, MSD, Pfizer, Raffo, and Roche; and has received support for attending meetings and/or travel from Roche, AstraZeneca, Bristol Myers Squibb, Pfizer, and Merck KGaA.
Figures





References
-
- Galsky M.D., Chen G.J., Oh W.K., Bellmunt J., Roth B.J., Petrioli R., Dogliotti L., Dreicer R., Sonpavde G. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann. Oncol. 2012;23:406–410. - PubMed
-
- De Santis M., Bellmunt J., Mead G., Kerst J.M., Leahy M., Maroto P., Gil T., Marreaud S., Daugaard G., Skoneczna I., et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 2012;30:191–199. - PMC - PubMed
-
- Sternberg C.N., de Mulder P., Schornagel J.H., Theodore C., Fossa S.D., van Oosterom A.T., Witjes J.A., Spina M., van Groeningen C.J., Duclos B., et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur. J. Cancer. 2006;42:50–54. - PubMed
-
- Dash A., Galsky M.D., Vickers A.J., Serio A.M., Koppie T.M., Dalbagni G., Bochner B.H. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–513. - PubMed
-
- Galsky M.D., Hahn N.M., Rosenberg J., Sonpavde G., Hutson T., Oh W.K., Dreicer R., Vogelzang N., Sternberg C.N., Bajorin D.F., Bellmunt J. Treatment of patients with metastatic urothelial cancer "unfit" for Cisplatin-based chemotherapy. J. Clin. Oncol. 2011;29:2432–2438. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

