The cryo-EM structure of homotetrameric attachment glycoprotein from langya henipavirus
- PMID: 38280880
- PMCID: PMC10821904
- DOI: 10.1038/s41467-024-45202-5
The cryo-EM structure of homotetrameric attachment glycoprotein from langya henipavirus
Abstract
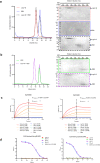
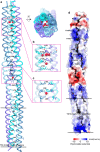
Langya Henipavirus (LayV) infection is an emerging zoonotic disease that has been causing respiratory symptoms in China since 2019. For virus entry, LayV's genome encodes the fusion protein F and the attachment glycoprotein G. However, the structural and functional information regarding LayV-G remains unclear. In this study, we revealed that LayV-G cannot bind to the receptors found in other HNVs, such as ephrin B2/B3, and it shows different antigenicity from HeV-G and NiV-G. Furthermore, we determined the near full-length structure of LayV-G, which displays a distinct mushroom-shaped configuration, distinguishing it from other attachment glycoproteins of HNV. The stalk and transmembrane regions resemble the stem and root of mushroom and four downward-tilted head domains as mushroom cap potentially interact with the F protein and influence membrane fusion process. Our findings enhance the understanding of emerging HNVs that cause human diseases through zoonotic transmission and provide implication for LayV related vaccine development.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Structure and design of Langya virus glycoprotein antigens.Proc Natl Acad Sci U S A. 2024 Apr 16;121(16):e2314990121. doi: 10.1073/pnas.2314990121. Epub 2024 Apr 9. Proc Natl Acad Sci U S A. 2024. PMID: 38593070 Free PMC article.
-
Prefusion stabilization of the Hendra and Langya virus F proteins.J Virol. 2024 Feb 20;98(2):e0137223. doi: 10.1128/jvi.01372-23. Epub 2024 Jan 12. J Virol. 2024. PMID: 38214525 Free PMC article.
-
Structure and antigenicity of divergent Henipavirus fusion glycoproteins.Nat Commun. 2023 Jun 16;14(1):3577. doi: 10.1038/s41467-023-39278-8. Nat Commun. 2023. PMID: 37328468 Free PMC article.
-
Infection and transmission of henipavirus in animals.Comp Immunol Microbiol Infect Dis. 2024 Jun;109:102183. doi: 10.1016/j.cimid.2024.102183. Epub 2024 Apr 17. Comp Immunol Microbiol Infect Dis. 2024. PMID: 38640700 Review.
-
Henipavirus mediated membrane fusion, virus entry and targeted therapeutics.Viruses. 2012 Feb;4(2):280-308. doi: 10.3390/v4020280. Epub 2012 Feb 13. Viruses. 2012. PMID: 22470837 Free PMC article. Review.
Cited by
-
Cryo-electron microscopy in the study of virus entry and infection.Front Mol Biosci. 2024 Jul 24;11:1429180. doi: 10.3389/fmolb.2024.1429180. eCollection 2024. Front Mol Biosci. 2024. PMID: 39114367 Free PMC article. Review.
-
Detection and characterization of Langya virus in Crocidura lasiura (the Ussuri white-toothed shrew), Republic of Korea.One Health. 2025 Mar 19;20:101017. doi: 10.1016/j.onehlt.2025.101017. eCollection 2025 Jun. One Health. 2025. PMID: 40212663 Free PMC article.
-
Henipaviruses: epidemiology, ecology, disease, and the development of vaccines and therapeutics.Clin Microbiol Rev. 2025 Mar 13;38(1):e0012823. doi: 10.1128/cmr.00128-23. Epub 2024 Dec 23. Clin Microbiol Rev. 2025. PMID: 39714175 Free PMC article. Review.
-
Structural and antigenic characterization of novel and diverse Henipavirus glycoproteins.bioRxiv [Preprint]. 2025 Apr 10:2024.12.11.627382. doi: 10.1101/2024.12.11.627382. bioRxiv. 2025. PMID: 39713338 Free PMC article. Preprint.
-
A Comparative Assessment of the Pathogenic Potential of Newly Discovered Henipaviruses.Pathogens. 2024 Jul 16;13(7):587. doi: 10.3390/pathogens13070587. Pathogens. 2024. PMID: 39057814 Free PMC article. Review.
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

