Re-expression of epigenetically silenced PTPRR by histone acetylation sensitizes RAS-mutant lung adenocarcinoma to SHP2 inhibition
- PMID: 38280930
- PMCID: PMC11073200
- DOI: 10.1007/s00018-023-05034-w
Re-expression of epigenetically silenced PTPRR by histone acetylation sensitizes RAS-mutant lung adenocarcinoma to SHP2 inhibition
Abstract
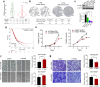
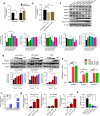
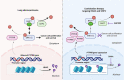
Silenced protein tyrosine phosphatase receptor type R (PTPRR) participates in mitogen-activated protein kinase (MAPK) signaling cascades during the genesis and development of tumors. Rat sarcoma virus (Ras) genes are frequently mutated in lung adenocarcinoma, thereby resulting in hyperactivation of downstream MAPK signaling. However, the molecular mechanism manipulating the regulation and function of PTPRR in RAS-mutant lung adenocarcinoma is not known. Patient records collected from the Cancer Genome Atlas and Gene Expression Omnibus showed that silenced PTPRR was positively correlated with the prognosis. Exogenous expression of PTPRR suppressed the proliferation and migration of lung cancer cells. PTPRR expression and Src homology 2 containing protein tyrosine phosphatase 2 (SHP2) inhibition acted synergistically to control ERK1/2 phosphorylation in RAS-driven lung cancer cells. Chromatin immunoprecipitation assay revealed that HDAC inhibition induced enriched histone acetylation in the promoter region of PTPRR and recovered PTPRR transcription. The combination of the HDAC inhibitor SAHA and SHP2 inhibitor SHP099 suppressed the progression of lung cancer markedly in vitro and in vivo. Therefore, we revealed the epigenetic silencing mechanism of PTPRR and demonstrated that combination therapy targeting HDAC and SHP2 might represent a novel strategy to treat RAS-mutant lung cancer.
Keywords: Anti-cancer drugs; Drug combination; Epigenetics; Signaling pathway; Targeted therapy.
© 2023. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

