A global survey of small RNA interactors identifies KhpA and KhpB as major RNA-binding proteins in Fusobacterium nucleatum
- PMID: 38281181
- PMCID: PMC11039985
- DOI: 10.1093/nar/gkae010
A global survey of small RNA interactors identifies KhpA and KhpB as major RNA-binding proteins in Fusobacterium nucleatum
Abstract
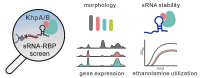
The common oral microbe Fusobacterium nucleatum has recently drawn attention after it was found to colonize tumors throughout the human body. Fusobacteria are also interesting study systems for bacterial RNA biology as these early-branching species encode many small noncoding RNAs (sRNAs) but lack homologs of the common RNA-binding proteins (RBPs) CsrA, Hfq and ProQ. To search for alternate sRNA-associated RBPs in F. nucleatum, we performed a systematic mass spectrometry analysis of proteins that co-purified with 19 different sRNAs. This approach revealed strong enrichment of the KH domain proteins KhpA and KhpB with nearly all tested sRNAs, including the σE-dependent sRNA FoxI, a regulator of several envelope proteins. KhpA/B act as a dimer to bind sRNAs with low micromolar affinity and influence the stability of several of their target transcripts. Transcriptome studies combined with biochemical and genetic analyses suggest that KhpA/B have several physiological functions, including being required for ethanolamine utilization. Our RBP search and the discovery of KhpA/B as major RBPs in F. nucleatum are important first steps in identifying key players of post-transcriptional control at the root of the bacterial phylogenetic tree.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

