H2A.Z is involved in premature aging and DSB repair initiation in muscle fibers
- PMID: 38281187
- PMCID: PMC11014257
- DOI: 10.1093/nar/gkae020
H2A.Z is involved in premature aging and DSB repair initiation in muscle fibers
Abstract
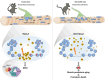
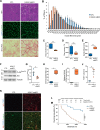
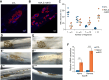
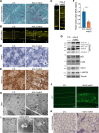
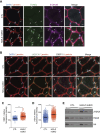
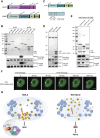
Histone variants are key epigenetic players, but their functional and physiological roles remain poorly understood. Here, we show that depletion of the histone variant H2A.Z in mouse skeletal muscle causes oxidative stress, oxidation of proteins, accumulation of DNA damages, and both neuromuscular junction and mitochondria lesions that consequently lead to premature muscle aging and reduced life span. Investigation of the molecular mechanisms involved shows that H2A.Z is required to initiate DNA double strand break repair by recruiting Ku80 at DNA lesions. This is achieved via specific interactions of Ku80 vWA domain with H2A.Z. Taken as a whole, our data reveal that H2A.Z containing nucleosomes act as a molecular platform to bring together the proteins required to initiate and process DNA double strand break repair.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

