Targeting MDM2 in malignancies is a promising strategy for overcoming resistance to anticancer immunotherapy
- PMID: 38281981
- PMCID: PMC10823613
- DOI: 10.1186/s12929-024-01004-x
Targeting MDM2 in malignancies is a promising strategy for overcoming resistance to anticancer immunotherapy
Abstract
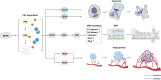
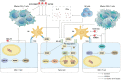
MDM2 has been established as a biomarker indicating poor prognosis for individuals undergoing immune checkpoint inhibitor (ICI) treatment for different malignancies by various pancancer studies. Specifically, patients who have MDM2 amplification are vulnerable to the development of hyperprogressive disease (HPD) following anticancer immunotherapy, resulting in marked deleterious effects on survival rates. The mechanism of MDM2 involves its role as an oncogene during the development of malignancy, and MDM2 can promote both metastasis and tumor cell proliferation, which indirectly leads to disease progression. Moreover, MDM2 is vitally involved in modifying the tumor immune microenvironment (TIME) as well as in influencing immune cells, eventually facilitating immune evasion and tolerance. Encouragingly, various MDM2 inhibitors have exhibited efficacy in relieving the TIME suppression caused by MDM2. These results demonstrate the prospects for breakthroughs in combination therapy using MDM2 inhibitors and anticancer immunotherapy.
Keywords: Anticancer immunotherapy; MDM2; Malignancies; Tumor immune microenvironment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
MDM2 inhibitor APG-115 synergizes with PD-1 blockade through enhancing antitumor immunity in the tumor microenvironment.J Immunother Cancer. 2019 Nov 28;7(1):327. doi: 10.1186/s40425-019-0750-6. J Immunother Cancer. 2019. PMID: 31779710 Free PMC article.
-
MDM2 Inhibition Enhances Immune Checkpoint Inhibitor Efficacy by Increasing IL15 and MHC Class II Production.Mol Cancer Res. 2023 Aug 1;21(8):849-864. doi: 10.1158/1541-7786.MCR-22-0898. Mol Cancer Res. 2023. PMID: 37071397 Free PMC article.
-
Hyperprogressive disease in patients receiving immune checkpoint inhibitors.Curr Probl Cancer. 2021 Jun;45(3):100688. doi: 10.1016/j.currproblcancer.2020.100688. Epub 2020 Dec 9. Curr Probl Cancer. 2021. PMID: 33334611 Review.
-
MDM2 Inhibitors for Cancer Therapy: The Past, Present, and Future.Pharmacol Rev. 2024 May 2;76(3):414-453. doi: 10.1124/pharmrev.123.001026. Pharmacol Rev. 2024. PMID: 38697854 Free PMC article. Review.
-
MDM2/X inhibitors under clinical evaluation: perspectives for the management of hematological malignancies and pediatric cancer.J Hematol Oncol. 2017 Jul 3;10(1):133. doi: 10.1186/s13045-017-0500-5. J Hematol Oncol. 2017. PMID: 28673313 Free PMC article. Review.
Cited by
-
Regulation of p53 by the mitotic surveillance/stopwatch pathway: implications in neurodevelopment and cancer.Front Cell Dev Biol. 2024 Sep 27;12:1451274. doi: 10.3389/fcell.2024.1451274. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39398482 Free PMC article. Review.
-
Primary Cardiac Intimal Sarcoma: Multi-Layered Strategy and Core Role of MDM2 Amplification/Co-Amplification and MDM2 Immunostaining.Diagnostics (Basel). 2024 Apr 28;14(9):919. doi: 10.3390/diagnostics14090919. Diagnostics (Basel). 2024. PMID: 38732333 Free PMC article. Review.
-
Bionic Power Play: Dual-Targeting MDMX/MDM2 to Reboot p53 to Beat Lung Adenocarcinoma's Immune Tricks.Int J Nanomedicine. 2025 Aug 13;20:9885-9897. doi: 10.2147/IJN.S533208. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40827233 Free PMC article.
References
-
- Sun D, Qian H, Wang J, Xie T, Teng F, Li J, Xing P. ARID1A deficiency reverses the response to anti-PD(L)1 therapy in EGFR-mutant lung adenocarcinoma by enhancing autophagy-inhibited type I interferon production. Cell Commun Signal. 2022;20(1):156. doi: 10.1186/s12964-022-00958-5. - DOI - PMC - PubMed
-
- Leighl NB, Hellmann MD, Hui R, Carcereny E, Felip E, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L, Patnaik A, Gubens M, Ramalingam SS, Lubiniecki GM, Zhang J, Piperdi B, Garon EB. Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Respir Med. 2019;7(4):347–357. doi: 10.1016/S2213-2600(18)30500-9. - DOI - PubMed
-
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Vandormael K, Riccio A, Yang J, Pietanza MC, Brahmer JR. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–546. doi: 10.1200/JCO.18.00149. - DOI - PubMed
-
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G, KEYNOTE-042 Investigators Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

