Evolving standards and future directions for systemic therapies in cervical cancer
- PMID: 38282261
- PMCID: PMC10948986
- DOI: 10.3802/jgo.2024.35.e65
Evolving standards and future directions for systemic therapies in cervical cancer
Abstract
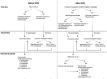
Several groundbreaking clinical trials with the potential to transform the management paradigm of both locally advanced and persistent, recurrent, or metastatic cervical cancers have been presented in 2023. This review describes the reported data from INTERLACE and KEYNOTE-A18 in the locally advanced setting, as well as BEATcc, innovaTV 301 and DESTINY-PanTumor02 for advanced disease. The practice implications of their positive results are interpreted in the context of global health considerations, and updated treatment algorithms are proposed. Furthermore, emerging trends in drug development for cervical cancer are discussed. As the routine use of immune checkpoint inhibitors (ICIs) for curative and palliative indications increases in the foreseeable future, patients whose cervical cancers which persist, relapse or progress after prior ICI exposure will represent an area of unmet clinical need and form the key target population for next-generation trials. Future research will help shape oncologists' approaches in the optimal selection, sequencing and re-treatment or rechallenge of immuno-oncology agents and/or antibody-drug conjugates in women with cervical cancer.
Keywords: Adoptive Immunotherapy; Cervical Cancer; Chemoradiotherapy; Immune Checkpoint Inhibitors; Immunoconjugates.
© 2024. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology, and Japan Society of Gynecologic Oncology.
Conflict of interest statement
A.D.J.M. has no conflict of interest to declare.
Figures


References
-
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149:778–789. - PubMed
-
- NCI Press Office. NCI issues clinical announcement on cervical cancer: chemotherapy plus radiation improves survival [Internet] Bethesda, MD: National Cancer Institute; 1999. [cited 2023 Dec 13]. Available from: http://www.nih.gov/news/pr/feb99/nci-22.htm.
-
- Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. - PubMed
-
- Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

