Dissecting EXP2 sequence requirements for protein export in malaria parasites
- PMID: 38282616
- PMCID: PMC10811066
- DOI: 10.3389/fcimb.2023.1332146
Dissecting EXP2 sequence requirements for protein export in malaria parasites
Abstract
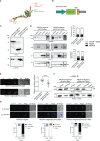
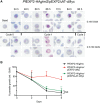
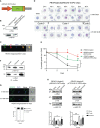
Apicomplexan parasites that reside within a parasitophorous vacuole harbor a conserved pore-forming protein that enables small-molecule transfer across the parasitophorous vacuole membrane (PVM). In Plasmodium parasites that cause malaria, this nutrient pore is formed by EXP2 which can complement the function of GRA17, an orthologous protein in Toxoplasma gondii. EXP2, however, has an additional function in Plasmodium parasites, serving also as the pore-forming component of the protein export machinery PTEX. To examine how EXP2 can play this additional role, transgenes that encoded truncations of EXP2, GRA17, hybrid GRA17-EXP2, or EXP2 under the transcriptional control of different promoters were expressed in EXP2 knockdown parasites to determine which could complement EXP2 function. This revealed that EXP2 is a unique pore-forming protein, and its protein export role in P. falciparum cannot be complemented by T. gondii GRA17. This was despite the addition of the EXP2 assembly strand and part of the linker helix to GRA17, which are regions necessary for the interaction of EXP2 with the other core PTEX components. This indicates that the body region of EXP2 plays a critical role in PTEX assembly and/or that the absence of other T. gondii GRA proteins in P. falciparum leads to its reduced efficiency of insertion into the PVM and complementation potential. Altering the timing and abundance of EXP2 expression did not affect protein export but affected parasite viability, indicating that the unique transcriptional profile of EXP2 when compared to other PTEX components enables it to serve an additional role in nutrient exchange.
Keywords: EXP2; GRA17; PTEX; Plasmodium; nutrient exchange; protein export.
Copyright © 2024 Pitman, Counihan, Modak, Chowdury, Gilson, Webb and de Koning-Ward.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
-
- Brown G. V., Culvenor J. G., Crewther P. E., Bianco A. E., Coppel R. L., Saint R. B., et al. . (1985). Localization of the ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum in merozoites and ring-infected erythrocytes. J. Exp. Med. 162, 774–779. doi: 10.1084/jem.162.2.774 - DOI - PMC - PubMed
-
- Bullen H. E., Charnaud S. C., Kalanon M., Riglar D. T., Dekiwadia C., Kangwanrangsan N., et al. . (2012). Biosynthesis, localisation and macromolecular arrangement of the Plasmodium falciparum translocon of exported proteins; PTEX. J. Biol. Chem. 287, 7871–7884. doi: 10.1074/jbc.M111.328591 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

