Virgin coconut oil (VCO) supplementation relieves symptoms and inflammation among COVID-19 positive adults: a single-blind randomized trial
- PMID: 38282651
- PMCID: PMC10808878
- DOI: 10.1017/jns.2023.118
Virgin coconut oil (VCO) supplementation relieves symptoms and inflammation among COVID-19 positive adults: a single-blind randomized trial
Abstract
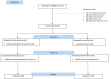
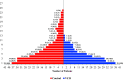
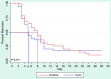
A clinical study conducted in 2020 showed that virgin coconut oil (VCO) has been found effective in the rapid relief of COVID-19 symptoms and normalization of the C-reactive protein (CRP) concentration among probable and suspected cases of COVID-19. This present study aimed to validate those results and to evaluate the effects of VCO among COVID-19 patients through a 28-day randomized, single-blind trial conducted among 76 SARS-CoV-2 RT-PCR (reverse transcription-polymerase chain report)-confirmed adults, with VCO given as a COVID-19 adjunct therapy. The results showed that VCO recipients were free from symptoms and had normal CRP concentrations by day 14. In comparison, participants in the control group reported relief from signs and symptoms on day 23, with normal CRP concentrations on day 25. This second study bolsters the use of VCO as an effective adjunct therapy for COVID-19-positive patients showing mild-to-moderate symptoms.
Keywords: C-reactive protein (CRP); COVID-19; Symptomatic relief; Virgin coconut oil (VCO).
© Published by Cambridge University Press on behalf of The Nutrition Society 2024.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

