High Energy Density Large Particle LiFePO4
- PMID: 38282686
- PMCID: PMC10810161
- DOI: 10.1021/acs.chemmater.3c02301
High Energy Density Large Particle LiFePO4
Abstract
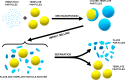
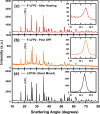
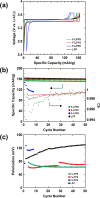
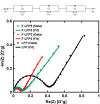
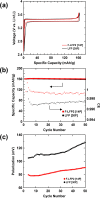
To improve the energy density of LiFePO4 (LFP) cathode materials for Li-ion cells, we have utilized a modified mechanofusion method for preparing micrometer-sized LFP/C composite flake particles. The resulting flake particle morphology resulted in improved packing efficiency, enabling an electrode porosity of 14% to be achieved at high loadings, which represents a volumetric energy density increase of 28% compared to conventional LFP. Furthermore, LFP/C flake composites electrodes were found to have a higher coulombic efficiency, a reduced voltage-polarization, and a greatly reduced charge transfer resistance compared to conventional LFP electrodes. This is believed to be due to the low surface area of the LFP/C flake composite particles coupled to fast Li+ ion grain boundary diffusion. The ability to make highly dense LFP and low surface area electrodes could have profound impacts, allowing for Li-ion cells to be made with low cost and low environmental impact LFP, while high achieving volumetric energy densities and high coulombic efficiencies.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Zheng L.; Hatchard T. D.; Obrovac M. N. A High-Quality Mechanofusion Coating for Enhancing Lithium-Ion Battery Cathode Material Performance. MRS Commun. 2019, 9 (1), 245–250. 10.1557/mrc.2018.209. - DOI
-
- Ren X.; Li Z.; Zheng Y.; Tian W.; Zhang K.; Cao J.; Tian S.; Guo J.; Wen L.; Liang G. High Volumetric Energy Density of LiFePO 4 Battery Based on Ultrasonic Vibration Combined with Thermal Drying Process. J. Electrochem. Soc. 2020, 167 (13), 130523. 10.1149/1945-7111/abba64. - DOI
-
- Padhi A. K.; Nanjundaswamy K. S.; Goodenough J. B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144 (4), 1188–1194. 10.1149/1.1837571. - DOI
-
- Satyavani T. V. S. L.; Srinivas Kumar A.; Subba Rao P. S. V. Methods of Synthesis and Performance Improvement of Lithium Iron Phosphate for High Rate Li-Ion Batteries: A Review. Engineering Science and Technology, an International Journal 2016, 19 (1), 178–188. 10.1016/j.jestch.2015.06.002. - DOI
-
- Franger S.; Benoit C.; Bourbon C.; Le Cras F. Chemistry and Electrochemistry of Composite LiFePO4Materials for Secondary Lithium Batteries. J. Phys. Chem. Solids 2006, 67 (5–6), 1338–1342. 10.1016/j.jpcs.2006.01.066. - DOI
LinkOut - more resources
Full Text Sources
