Long-term stability of clinical-grade lentiviral vectors for cell therapy
- PMID: 38282894
- PMCID: PMC10811425
- DOI: 10.1016/j.omtm.2024.101186
Long-term stability of clinical-grade lentiviral vectors for cell therapy
Abstract
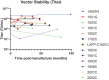
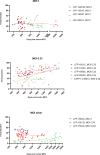
The use of lentiviral vectors in cell and gene therapy is steadily increasing, both in commercial and investigational therapies. Although existing data increasingly support the usefulness and safety of clinical-grade lentiviral vectors used in cell manufacturing, comprehensive studies specifically addressing their long-term stability are currently lacking. This is significant considering the high cost of producing and testing GMP-grade vectors, the limited number of production facilities, and lengthy queue for production slots. Therefore, an extended shelf life is a critical attribute to justify the investment in large vector lots for investigational cell therapies. This study offers a thorough examination of essential stability attributes, including vector titer, transduction efficiency, and potency for a series of clinical-grade vector lots, each assessed at a minimum of 36 months following their date of manufacture. The 13 vector lots included in this study were used for cell product manufacturing in 16 different clinical trials, and at the time of the analysis had a maximum storage time at -80°C of up to 8 years. The results emphasize the long-term durability and efficacy of GMP-grade lentiviral vectors for use in ex vivo cell therapy manufacturing.
Keywords: cell therapy; lentiviral vector; potency; titer stability; transduction efficiency.
© 2024 The Author(s).
Conflict of interest statement
C.H.J is an inventor on patents and/or patent applications licensed to Novartis Institutes of Biomedical Research and receives license revenue from such licenses. C.H.J. is a scientific cofounder of Capstan Therapeutics, Bluewhale Bio, and Tceleron; is a consultant to Kite Pharma; and is a member of the Scientific Advisory Boards of AC Immune, Alaunos, BluesphereBio, Cabaletta, Carisma, Cartography Biosciences, Cellares, Celldex, Decheng, Poseida, Replay Bio, Verismo, and WIRB-Copernicus Group. A.C. is a scientific cofounder of Tceleron, and is a consultant to Kite Pharma and Bluewhale Bio. J.A.F. has received grants and personal fees from Cartography Biosciences, grants from Tmunity Therapeutics, and personal fees from Retro Bio and Shennon Bio outside the submitted work. In addition, J.A.F. holds patents related to CAR T cells for cancer that are licensed and associated with royalties.
Figures
References
-
- Comisel R.-M., Kara B., Fiesser F.H., Farid S.S. Lentiviral vector bioprocess economics for cell and gene therapy commercialization. Biochem. Eng. J. 2021;167
-
- Gene Therapy Clinical Trials Worldwide . Wiley; 2023. Provided by the Journal of Gene Medicine.
-
- Bear A.S., Morgan R.A., Cornetta K., June C.H., Binder-Scholl G., Dudley M.E., Feldman S.A., Rosenberg S.A., Shurtleff S.A., Rooney C.M., et al. Replication-competent retroviruses in gene-modified T cells used in clinical trials: is it time to revise the testing requirements? Mol. Ther. 2012;20:246–249. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

