Targeted chemotherapy via HER2-based chimeric antigen receptor (CAR) engineered T-cell membrane coated polymeric nanoparticles
- PMID: 38282968
- PMCID: PMC10821609
- DOI: 10.1016/j.bioactmat.2023.12.027
Targeted chemotherapy via HER2-based chimeric antigen receptor (CAR) engineered T-cell membrane coated polymeric nanoparticles
Abstract
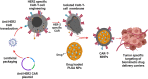
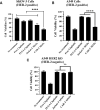
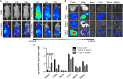
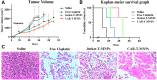
Cell membrane-derived nanoparticles (NPs) have recently gained popularity due to their desirable features in drug delivery such as mimicking properties of native cells, impeding systemic clearance, and altering foreign body responses. Besides NP technology, adoptive immunotherapy has emerged due to its promise in cancer specificity and therapeutic efficacy. In this research, we developed a biomimetic drug carrier based on chimeric antigen receptor (CAR) transduced T-cell membranes. For that purpose, anti-HER2 CAR-T cells were engineered via lentiviral transduction of anti-HER2 CAR coding lentiviral plasmids. Anti-HER2 CAR-T cells were characterized by their specific activities against the HER2 antigen and used for cell membrane extraction. Anti-cancer drug Cisplatin-loaded poly (D, l-lactide-co-glycolic acid) (PLGA) NPs were coated with anti-human epidermal growth factor receptor 2 (HER2)-specific CAR engineered T-cell membranes. Anti-HER2 CAR-T-cell membrane-coated PLGA NPs (CAR-T-MNPs) were characterized and confirmed via fluorescent microscopy and flow cytometry. Membrane-coated NPs showed a sustained drug release over the course of 21 days in physiological conditions. Cisplatin-loaded CAR-T-MNPs also inhibited the growth of multiple HER2+ cancer cells in vitro. In addition, in vitro uptake studies revealed that CAR-T-MNPs showed an increased uptake by A549 cells. These results were also confirmed via in vivo biodistribution and therapeutic studies using a subcutaneous lung cancer model in nude mice. CAR-T-MNPs localized preferentially at tumor areas compared to those of other studied groups and consisted of a significant reduction in tumor growth in tumor-bearing mice. In Conclusion, the new CAR modified cell membrane-coated NP drug-delivery platform has demonstrated its efficacy both in vitro and in vivo. Therefore, CAR engineered membrane-coated NP system could be a promising cell-mimicking drug carrier that could improve therapeutic outcomes of lung cancer treatments.
Keywords: CAR-T cells; Cancer chemotherapy; Membrane-based drug delivery; Nanoparticles.
© 2024 The Authors.
Conflict of interest statement
There is no Conflict of Interest. The co-authors have approved the manuscript submission.
Figures






References
-
- Athey V.L., Suckling R.J., Tod A.M., Walters S.J., Rogers T.K. Early diagnosis of lung cancer: evaluation of a community-based social marketing intervention. Thorax. 2012;67(5):412–417. - PubMed
-
- Fukuoka M., Yano S., Giaccone G., Tamura T., Nakagawa K., Douillard J.Y., Nishiwaki Y., Vansteenkiste J., Kudoh S., Rischin D., Eek R., Horai T., Noda K., Takata I., Smit E., Averbuch S., Macleod A., Feyereislova A., Dong R.P., Baselga J. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J. Clin. Oncol. 2003;21(12):2237–2246. - PubMed
-
- Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA A Cancer J. Clin. 2013;63(1):11–30. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

