Structure-based design, and development of amidinyl, amidoximyl and hydroxamic acid based organic molecules as novel antimalarial drug candidates
- PMID: 38283036
- PMCID: PMC10810238
- DOI: 10.1016/j.arabjc.2023.105573
Structure-based design, and development of amidinyl, amidoximyl and hydroxamic acid based organic molecules as novel antimalarial drug candidates
Abstract
Malaria remains a significant global health concern causing numerous fatalities and the emergence of antimalarial drug resistance highlights the urgent need for novel therapeutic options with innovative mechanisms of action and targets. This study aimed to design potential inhibitors of Plasmodium falciparum 6-pyruvoyltetrahydropterin synthase (PfPTPS), synthesize them, and experimentally validate their efficacy as antimalarial agents. A structure-based approach was employed to design a series of novel derivatives, including amidinyl, amidoximyl and hydroxamic acid analogs (1c, 1d, 2b, and 3b), with a focus on their ability to bind to the Zn2+ present in the active site of PfPTPS. The syntheses of these compounds were accomplished through various multi-step synthetic pathways and their structural identities were confirmed using 1H and 13C NMR spectra, mass spectra, and elemental analysis. The compounds were screened for their antiplasmodial activity against the NF54 strain of P. falciparum and in vitro cytotoxicity testing was performed using L-6 cells. The in vivo acute toxicity of the compounds was evaluated in mice. Docking studies of the compounds with the 3D structure of PfPTPS revealed their strong binding affinities, with compound 3b exhibiting notable metal-acceptor interaction with the Zn2+ in the protein binding pocket thereby positioning it as a lead compound for PfPTPS inhibition. The in vitro antiplasmodial studies revealed moderate efficacies against the Pf NF54 strain, particularly compounds 1d and 3b which displayed IC50 < 0.2 μM. No significant cytotoxicity was noted on the L-6 rat cell line. Moreover, in vivo studies suggested that compound 3b exhibited both safety and efficacy in treating rodent malaria. The identified lead compound in this study represents a possible candidate for antimalarial drug development and can be further explored in the search for alternative antifolate drugs to combat the malaria menace.
Keywords: Acute toxicity; Cytotoxicity; Hydroxamic acids; Malaria; Suppression.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
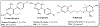






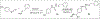
Similar articles
-
Phytochemical screening, antioxidant analyses, and in vitro and in vivo antimalarial activities of herbal medicinal plant - Rotheca serrata (L.) Steane & Mabb.J Ethnopharmacol. 2024 Mar 1;321:117466. doi: 10.1016/j.jep.2023.117466. Epub 2023 Nov 21. J Ethnopharmacol. 2024. PMID: 37981115
-
Phytochemical screening, cytotoxicity assessment and evaluation of in vitro antiplasmodial and in vivo antimalarial activities of Mentha spicata L. methanolic leaf extract.J Ethnopharmacol. 2022 Nov 15;298:115636. doi: 10.1016/j.jep.2022.115636. Epub 2022 Aug 23. J Ethnopharmacol. 2022. PMID: 35998785
-
In vitro antiplasmodial and cytotoxicity activities of crude extracts and major compounds from Goniothalamus lanceolatus.J Ethnopharmacol. 2020 May 23;254:112657. doi: 10.1016/j.jep.2020.112657. Epub 2020 Feb 8. J Ethnopharmacol. 2020. PMID: 32045683
-
A Structural Chemistry Perspective on the Antimalarial Properties of Thiosemicarbazone Metal Complexes.Mini Rev Med Chem. 2019;19(7):569-590. doi: 10.2174/1389557518666181015152657. Mini Rev Med Chem. 2019. PMID: 30324878 Review.
-
Redox modulating small molecules having antimalarial efficacy.Biochem Pharmacol. 2023 Dec;218:115927. doi: 10.1016/j.bcp.2023.115927. Epub 2023 Nov 20. Biochem Pharmacol. 2023. PMID: 37992998 Review.
Cited by
-
Insights into the Gene Expression Profile of Classical Hodgkin Lymphoma: A Study towards Discovery of Novel Therapeutic Targets.Molecules. 2024 Jul 25;29(15):3476. doi: 10.3390/molecules29153476. Molecules. 2024. PMID: 39124881 Free PMC article.
References
-
- Acuna VV, Hopper RM, Yoder RJ, 2020. Computer-Aided Drug Design for the Organic Chemistry Laboratory Using Accessible Molecular Modeling Tools. J. Chem. Educ 97, 760–763. 10.1021/acs.jchemed.9b00592. - DOI
-
- Adams L, Afiadenyo M, Kwofie SK, Wilson MD, Kusi KA, Obiri-Yeboah D, Moane S, McKeon-Bennett M, 2023. In silico screening of phytochemicals from Dissotis rotundifolia against Plasmodium falciparum Dihydrofolate Reductase. Phytomedicine Plus 3, 100447. 10.1016/j.phyplu.2023.100447. - DOI
-
- Adebiyi E, Ajani O, Klika K, 2021. Organic compounds and uses thereof EP4070856.
-
- Akinnusi PA, Olubode SO, Adebesin AO, Osadipe TJ, Nwankwo DO, Adebisi AD, Titilayo BAI, Alo YM, Owoloye A, Oyebola KM, 2023. Structure-based scoring of anthocyanins and molecular modeling of PfLDH, PfDHODH, and PfDHFR reveal novel potential P. falciparum inhibitors. Informatics Med. Unlocked 38, 101206. 10.1016/j.imu.2023.101206. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
