Role of Intravitreal Dexamethasone Implant in the Management of Treatment-Naive Diabetic Macular Edema: A Pre-Cataract Surgical Approach for Patients with Systemic Contraindications to Anti-VEGF Therapy
- PMID: 38283181
- PMCID: PMC10821638
- DOI: 10.2147/OPTH.S449250
Role of Intravitreal Dexamethasone Implant in the Management of Treatment-Naive Diabetic Macular Edema: A Pre-Cataract Surgical Approach for Patients with Systemic Contraindications to Anti-VEGF Therapy
Abstract
Purpose: Diabetic macular edema (DME) is a significant cause of vision impairment, posing challenges in its management due to variable responses and patient diversity. While anti-vascular endothelial growth factor (anti-VEGF) agents have revolutionized DME treatment, some patients are not suitable candidates for this therapy. Intravitreal corticosteroid therapy, such as the dexamethasone implant (DEX), has emerged as an alternative. This study aimed to comprehensively investigate the role of intravitreal DEX in treatment-naive DME patients with systemic contraindications to anti-VEGF therapy, administered one month before cataract surgery.
Patients and methods: A single-center retrospective study included 20 eyes with controlled diabetes, visually significant cataracts, untreated DME, and systemic contraindications for anti-VEGF therapy. Patients underwent DEX treatment followed by cataract surgery after one month. Best-corrected visual acuity (BCVA), central macular thickness (CMT), and intraocular pressure (IOP) were assessed at multiple time points.
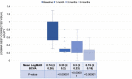
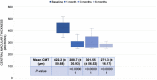
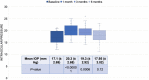
Results: BCVA significantly improved on days 30, 90, and 180 post-DEX (P<0.00001). CMT showed a significant decrease at day 30 (P<0.00001), which was sustained through days 90 and 180 (P<0.00001). Recurrent DME was observed in 25% of eyes on day 90. IOP increased significantly at days 30 (P<0.00001) and 90 (P=0.0006), returning to baseline by day 180. However, only two eyes needed topical anti-glaucoma treatment. No other ocular or systemic adverse events were noted.
Conclusion: Intravitreal DEX administered one month before cataract surgery offers a promising treatment strategy for treatment-naive DME patients with systemic contraindications to anti-VEGF therapy. The study's findings provide insights into improving visual acuity and reducing macular thickness, along with manageable IOP changes. This personalized approach is a valuable addition to DME management, especially for complex medical cases, warranting further research and consideration for clinical practice.
Keywords: Ozurdex; dexamethasone implant; diabetic macular edema.
© 2024 Chakraborty et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Dexamethasone intravitreal implant (Ozurdex) in diabetic macular edema: real-world data versus clinical trials outcomes.Int J Ophthalmol. 2021 Oct 18;14(10):1571-1580. doi: 10.18240/ijo.2021.10.15. eCollection 2021. Int J Ophthalmol. 2021. PMID: 34667735 Free PMC article.
-
A meta-analysis of the effect of a dexamethasone intravitreal implant versus intravitreal anti-vascular endothelial growth factor treatment for diabetic macular edema.BMC Ophthalmol. 2018 May 21;18(1):121. doi: 10.1186/s12886-018-0779-1. BMC Ophthalmol. 2018. PMID: 29784048 Free PMC article. Review.
-
Intravitreal steroids for macular edema in diabetes.Cochrane Database Syst Rev. 2020 Nov 17;11(11):CD005656. doi: 10.1002/14651858.CD005656.pub3. Cochrane Database Syst Rev. 2020. PMID: 33206392 Free PMC article.
-
Effect of dexamethasone intravitreal implant for refractory and treatment-naive diabetic macular edema in Taiwanese patients.J Chin Med Assoc. 2021 Mar 1;84(3):326-330. doi: 10.1097/JCMA.0000000000000483. J Chin Med Assoc. 2021. PMID: 33433135
-
Comparative Analysis of Intravitreal Dexamethasone Implant (Ozurdex) and Brolucizumab Injection in the Treatment of Diabetic Macular Edema with Hyperreflective Intraretinal Dots: A Retrospective Study.Clin Ophthalmol. 2024 Oct 15;18:2897-2905. doi: 10.2147/OPTH.S484731. eCollection 2024. Clin Ophthalmol. 2024. PMID: 39429440 Free PMC article.
Cited by
-
Dexamethasone implant for refractory macular edema secondary to diabetic retinopathy and retinal vein occlusion.Int J Ophthalmol. 2024 Oct 18;17(10):1837-1842. doi: 10.18240/ijo.2024.10.09. eCollection 2024. Int J Ophthalmol. 2024. PMID: 39430031 Free PMC article.
References
-
- Bandello F, Battaglia Parodi M, Lanzetta P, et al. Diabetic macular edema. Dev Ophthalmol. 2017;58:102–138. - PubMed
LinkOut - more resources
Full Text Sources

