Impact of dipeptide on ADC physicochemical properties and efficacy identifies Ala-Ala as the optimal dipeptide
- PMID: 38283215
- PMCID: PMC10809321
- DOI: 10.1039/d3md00473b
Impact of dipeptide on ADC physicochemical properties and efficacy identifies Ala-Ala as the optimal dipeptide
Abstract
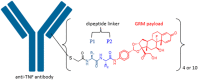
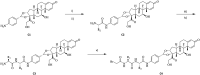
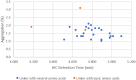
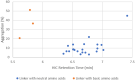
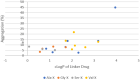
Side chains of natural occurring amino acids vary greatly in terms of charge state, polarity, size and hydrophobicity. Using a linear synthetic route, two amino acids were sequentially coupled to a potent glucocorticoid receptor modulator (GRM) to afford a library of dipeptide-GRM linker payloads with a range of in silico properties. The linker payloads were conjugated to a mouse anti-TNF antibody through interchain disulfide Cys. Impact of various dipeptide linkers on ADC physical properties, including solubility, hydrophobicity, and aggregation were evaluated and the in silico properties pI, Log P and tPSA of the linker drugs used to correlate with these properties. ADCs were screened in a GRE luciferase reporter assay to compare their in vitro efficacy. Data identified Ala-Ala as a superior dipeptide linker that allowed a maximum drug load of 10 while affording ADCs with low aggregation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
The medicinal chemistry evolution of antibody-drug conjugates.RSC Med Chem. 2024 Feb 28;15(3):809-831. doi: 10.1039/d3md00674c. eCollection 2024 Mar 20. RSC Med Chem. 2024. PMID: 38516594 Free PMC article. Review.
-
Optimizing Lysosomal Activation of Antibody-Drug Conjugates (ADCs) by Incorporation of Novel Cleavable Dipeptide Linkers.Mol Pharm. 2019 Dec 2;16(12):4817-4825. doi: 10.1021/acs.molpharmaceut.9b00696. Epub 2019 Oct 29. Mol Pharm. 2019. PMID: 31609629
-
Antibody drug conjugates beyond cytotoxic payloads.Prog Med Chem. 2023;62:1-59. doi: 10.1016/bs.pmch.2023.10.001. Epub 2023 Nov 14. Prog Med Chem. 2023. PMID: 37981349
-
Discovery of ABBV-3373, an Anti-TNF Glucocorticoid Receptor Modulator Immunology Antibody Drug Conjugate.J Med Chem. 2022 Dec 8;65(23):15893-15934. doi: 10.1021/acs.jmedchem.2c01579. Epub 2022 Nov 17. J Med Chem. 2022. PMID: 36394224
-
[Novel Chemical Linkers for Next-generation Antibody-drug Conjugates(ADCs)].Yakugaku Zasshi. 2019;139(2):209-219. doi: 10.1248/yakushi.18-00169-3. Yakugaku Zasshi. 2019. PMID: 30713230 Review. Japanese.
Cited by
-
The medicinal chemistry evolution of antibody-drug conjugates.RSC Med Chem. 2024 Feb 28;15(3):809-831. doi: 10.1039/d3md00674c. eCollection 2024 Mar 20. RSC Med Chem. 2024. PMID: 38516594 Free PMC article. Review.
-
Benzyl Ammonium Carbamates Undergo Two-Step Linker Cleavage and Improve the Properties of Antibody Conjugates.Angew Chem Int Ed Engl. 2025 Feb 3;64(6):e202417651. doi: 10.1002/anie.202417651. Epub 2024 Dec 18. Angew Chem Int Ed Engl. 2025. PMID: 39696914 Free PMC article.
References
-
- Polson A. G. Calemine-Fenaux J. Chan P. Chang W. Christensen E. Clark S. Sauvage F. J. Eaton D. Elkins K. Elliott J. M. Frantz G. Fuji R. N. Gray A. Harden K. Ingle G. S. Kljavin N. M. Koeppen H. Nelson C. Prabhu S. Raab H. Ross S. Slaga D. S. Stephan J. Scales S. J. Spencer S. D. Vandlen R. Wranik B. Yu S. Zheng B. Ebens A. Antibody-drug conjugates for the treatment of non-Hodgkin's lymphoma: target and linker-drug selection. Cancer Res. 2009;69:2358–2364. doi: 10.1158/0008-5472.CAN-08-2250. - DOI - PubMed
-
- Dubowchik G. M. Firestone R. A. Padilla L. Willner D. Hofstead S. J. Mosure K. Knipe J. O. Lasch S. J. Trail P. A. Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjugate Chem. 2002;13:855–869. doi: 10.1021/bc025536j. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

