Evaluating Personalized (N-of-1) Trials in Rare Diseases: How Much Experimentation Is Enough?
- PMID: 38283317
- PMCID: PMC10813653
- DOI: 10.1162/99608f92.e11adff0
Evaluating Personalized (N-of-1) Trials in Rare Diseases: How Much Experimentation Is Enough?
Abstract
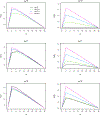
For rare diseases, conducting large, randomized trials of new treatments can be infeasible due to limited sample size, and it may answer the wrong scientific questions due to heterogeneity of treatment effects. Personalized (N-of-1) trials are multi-period crossover studies that aim to estimate individual treatment effects, thereby identifying the optimal treatments for individuals. This article examines the statistical design issues of evaluating a personalized (N-of-1) treatment program in people with amyotrophic lateral sclerosis (ALS). We propose an evaluation framework based on an analytical model for longitudinal data observed in a personalized trial. Under this framework, we address two design parameters: length of experimentation in each trial and number of trials needed. For the former, we consider patient-centric design criteria that aim to maximize the benefits of enrolled patients. Using theoretical investigation and numerical studies, we demonstrate that, from a patient's perspective, the duration of an experimentation period should be no longer than one-third of the entire follow-up period of the trial. For the latter, we provide analytical formulae to calculate the power for testing quality improvement due to personalized trials in a randomized evaluation program and hence determine the required number of trials needed for the program. We apply our theoretical results to design an evaluation program for ALS treatments informed by pilot data and show that the length of experimentation has a small impact on power relative to other factors such as the degree of heterogeneity of treatment effects.
Keywords: ALS; heterogeneity of treatment effects (HTE); minimally clinically important heterogeneity; patient-centered research; rare diseases; sample size formulae.
Figures
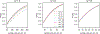
References
-
- Cheung K, Wood D, Zhang K, Ridenour TA, Derby L, St Onge T, Duan N, Duer-Hefele J, Davidson KW, Kronish IM, & Moise N. (2020). Personal preferences for personalized trials among patients with chronic experience: An empirical Bayesian analysis of a conjoint survey. BMJ Open, 10(6), Article e036056. 10.1136/bmjopen-2019-036056 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
