Thrombotic and Hemorrhagic Complications Following Left Ventricular Assist Device Placement: An Emphasis on Gastrointestinal Bleeding, Stroke, and Pump Thrombosis
- PMID: 38283491
- PMCID: PMC10811971
- DOI: 10.7759/cureus.51160
Thrombotic and Hemorrhagic Complications Following Left Ventricular Assist Device Placement: An Emphasis on Gastrointestinal Bleeding, Stroke, and Pump Thrombosis
Abstract
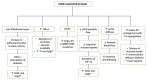
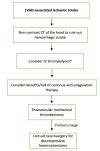
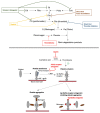
The left ventricular assist device (LVAD) is a mechanical circulatory support device that supports the heart failure patient as a bridge to transplant (BTT) or as a destination therapy for those who have other medical comorbidities or complications that disqualify them from meeting transplant criteria. In patients with severe heart failure, LVAD use has extended survival and improved signs and symptoms of cardiac congestion and low cardiac output, such as dyspnea, fatigue, and exercise intolerance. However, these devices are associated with specific hematologic and thrombotic complications. In this manuscript, we review the common hematologic complications of LVADs.
Keywords: device-related thrombus (drt); gastrointestinal bleeding; heart failure; hemorrhagic complications; left ventricular assist device; stroke; thrombotic complications.
Copyright © 2023, Phan et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Clinical implications of LDH isoenzymes in hemolysis and continuous-flow left ventricular assist device-induced thrombosis. Gordon JS, Wood CT, Luc JG, et al. Artif Organs. 2020;44:231–238. - PubMed
-
- 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Yancy CW, Jessup M, Bozkurt B, et al. J Am Coll Cardiol. 2013;62:0–239. - PubMed
-
- Stroke and intracranial hemorrhage in HeartMate II and HeartWare left ventricular assist devices: A systematic review. Cho SM, Moazami N, Frontera JA. Neurocrit Care. 2017;27:17–25. - PubMed
-
- Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Lietz K, Long JW, Kfoury AG, et al. Circulation. 2007;116:497–505. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
