AKR1B1 drives hyperglycemia-induced metabolic reprogramming in MASLD-associated hepatocellular carcinoma
- PMID: 38283757
- PMCID: PMC10820337
- DOI: 10.1016/j.jhepr.2023.100974
AKR1B1 drives hyperglycemia-induced metabolic reprogramming in MASLD-associated hepatocellular carcinoma
Abstract
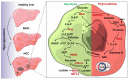
Background & aims: The mechanism behind the progressive pathological alteration in metabolic dysfunction-associated steatotic liver disease/steatohepatitis (MASLD/MASH)-associated hepatocellular carcinoma (HCC) is poorly understood. In the present study, we investigated the role of the polyol pathway enzyme AKR1B1 in metabolic switching associated with MASLD/MASH and in the progression of HCC.
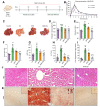
Methods: AKR1B1 expression was estimated in the tissue and plasma of patients with MASLD/MASH, HCC, and HCC with diabetes mellitus. The role of AKR1B1 in metabolic switching in vitro was assessed through media conditioning, lentiviral transfection, and pharmacological probes. A proteomic and metabolomic approach was applied for the in-depth investigation of metabolic pathways. Preclinically, mice were subjected to a high-fructose diet and diethylnitrosamine to investigate the role of AKR1B1 in the hyperglycemia-mediated metabolic switching characteristic of MASLD-HCC.
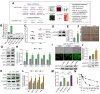
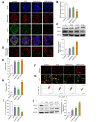
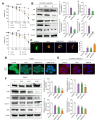
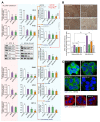
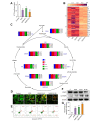
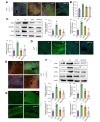
Results: A significant increase in the expression of AKR1B1 was observed in tissue and plasma samples from patients with MASLD/MASH, HCC, and HCC with diabetes mellitus compared to normal samples. Mechanistically, in vitro assays revealed that AKR1B1 modulates the Warburg effect, mitochondrial dynamics, the tricarboxylic acid cycle, and lipogenesis to promote hyperglycemia-mediated MASLD and cancer progression. A pathological increase in the expression of AKR1B1 was observed in experimental MASLD-HCC, and expression was positively correlated with high blood glucose levels. High-fructose diet + diethylnitrosamine-treated animals also exhibited statistically significant elevation of metabolic markers and carcinogenesis markers. AKR1B1 inhibition with epalrestat or NARI-29 inhibited cellular metabolism in in vitro and in vivo models.
Conclusions: Pathological AKR1B1 modulates hepatic metabolism to promote MASLD-associated hepatocarcinogenesis. Aldose reductase inhibition modulates the glycolytic pathway to prevent precancerous hepatocyte formation.
Impact and implications: This research work highlights AKR1B1 as a druggable target in metabolic dysfunction-associated steatotic liver disease (MASLD) and hepatocellular carcinoma (HCC), which could provide the basis for the development of new chemotherapeutic agents. Moreover, our results indicate the potential of plasma AKR1B1 levels as a prognostic marker and diagnostic test for MASLD and associated HCC. Additionally, a major observation in this study was that AKR1B1 is associated with the promotion of the Warburg effect in HCC.
Keywords: HCC; MASLD/MASH; Metabolism; NARI-29; Warburg effect; diethyl nitrosamine; epalrestat; high fructose diet.
© 2023 The Authors.
Conflict of interest statement
The authors of this study declare that they do not have any conflict of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- El-Serag E.B., Hampel H., Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

