Investigation of Corrosion Inhibition of Mild Steel in 0.5 M H2SO4 with Lachancea fermentati Inhibitor Extracted from Rotten Grapefruits (Vitis vinifera): Adsorption, Thermodynamic, Electrochemical, and Quantum Chemical Studies
- PMID: 38283783
- PMCID: PMC10811774
- DOI: 10.1021/acsphyschemau.3c00055
Investigation of Corrosion Inhibition of Mild Steel in 0.5 M H2SO4 with Lachancea fermentati Inhibitor Extracted from Rotten Grapefruits (Vitis vinifera): Adsorption, Thermodynamic, Electrochemical, and Quantum Chemical Studies
Abstract
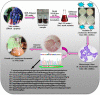
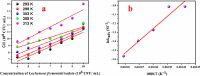
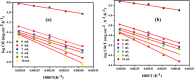
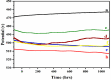
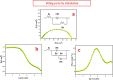
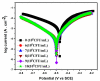
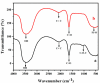
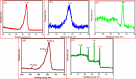
Corrosion inhibition of mild steel (MS) was studied using Lachancea fermentati isolate in 0.5 M H2SO4, which was isolated from rotten grapes (Vitis vinifera) via biofilm formation. Biofilm over the MS surface was asserted by employing FT-IR and FE-SEM with EDXS, electrochemical impedance spectroscopy (EIS), AFM, and DFT-ESP techniques. The weight loss experiments and temperature studies supported the physical adsorption behavior of the corrosion inhibitors. The maximum inhibition efficiency (IE) value (90%) was observed at 293 K for 9 × 106 cfu/mL of Lachancea fermentati isolate. The adsorption of Lachancea fermentati isolate on the surface of MS confirms Langmuir's adsorption isotherm model, and the -ΔG values indicate the spontaneous adsorption of inhibitor over the MS surface. Electrochemical studies, such as potentiodynamic polarization (PDP) and EIS were carried out to investigate the charge transfer (CT) reaction of the Lachancea fermentati isolate. Tafel polarization curves reveal that the Lachancea fermentati isolate acts as a mixed type of inhibitor. The Nyquist plots (EIS) indicate the increase in charge transfer resistance (Rct) and decrease of double-layer capacitance (Cdl) values when increasing the concentration of Lachancea fermentati isolate. The spectral studies, such as UV-vis and FT-IR, confirm the formation of a complex between MS and the Lachancea fermentati isolate inhibitor. The formation of biofilm on the MS surface was confirmed by FE-SEM, EDXS, and XPS analysis. The proposed bioinhibitor shows great potential for the corrosion inhibition of mild steel in acid media.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Fiori-Bimbi M. V.; Alvarez P. E.; Vaca H.; Gervasi C. A. Corrosion inhibition of mild steel in HCl solution by Pectin. Corros. Sci. 2015, 92, 192–199. 10.1016/j.corsci.2014.12.002. - DOI
-
- Alaneme K. K.; Olusegun S. J.; Adelowo O. T. Corrosion inhibition and adsorption mechanism studies of Hunteria umbellate seed husk extracts on mild steel immersed in acidic solutions. Alex. Eng. J. 2016, 55 (1), 673–681. 10.1016/j.aej.2015.10.009. - DOI
-
- Singh D. K.; Kumar S.; Udayabhanu G.; John R. P. 4(N,N-dimethylamino) benzaldehyde nicotinic hydrazone as corrosion inhibitor for mild steel in 1M HCl solution: An experimental and theoretical study. J. Mol. Liquid. 2016, 216, 738–746. 10.1016/j.molliq.2016.02.012. - DOI
-
- Koch G. H.; Brongers M. P. H.; Thompson N. G.; Virmani Y. P.; Payer J. H.. Corrosion costs and preventive strategies in the United States. Report FHWA-RD-01–156, 2003.
LinkOut - more resources
Full Text Sources
Miscellaneous
