Label-Free Biochemical Sensing Using Processed Optical Fiber Interferometry: A Review
- PMID: 38284054
- PMCID: PMC10809379
- DOI: 10.1021/acsomega.3c03970
Label-Free Biochemical Sensing Using Processed Optical Fiber Interferometry: A Review
Abstract
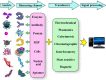
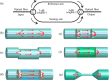
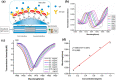
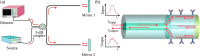
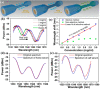
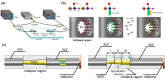
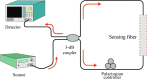
Over the last 20 years, optical fiber-based devices have been exploited extensively in the field of biochemical sensing, with applications in many specific areas such as the food processing industry, environmental monitoring, health diagnosis, bioengineering, disease diagnosis, and the drug industry due to their compact, label-free, and highly sensitive detection. The selective and accurate detection of biochemicals is an essential part of biosensing devices, which is to be done through effective functionalization of highly specific recognition agents, such as enzymes, DNA, receptors, etc., over the transducing surface. Among many optical fiber-based sensing technologies, optical fiber interferometry-based biosensors are one of the broadly used methods with the advantages of biocompatibility, compact size, high sensitivity, high-resolution sensing, lower detection limits, operating wavelength tunability, etc. This Review provides a comprehensive review of the fundamentals as well as the current advances in developing optical fiber interferometry-based biochemical sensors. In the beginning, a generic biosensor and its several components are introduced, followed by the fundamentals and state-of-art technology behind developing a variety of interferometry-based fiber optic sensors. These include the Mach-Zehnder interferometer, the Michelson interferometer, the Fabry-Perot interferometer, the Sagnac interferometer, and biolayer interferometry (BLI). Further, several technical reports are comprehensively reviewed and compared in a tabulated form for better comparison along with their advantages and disadvantages. Further, the limitations and possible solutions for these sensors are discussed to transform these in-lab devices into commercial industry applications. At the end, in conclusion, comments on the prospects of field development toward the commercialization of sensor technology are also provided. The Review targets a broad range of audiences including beginners and also motivates the experts helping to solve the real issues for developing an industry-oriented sensing device.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



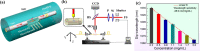




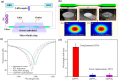
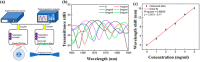





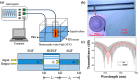
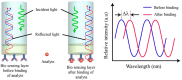
References
-
- Wang Y.; Zeng S.; Humbert G.; Ho H. P. Microfluidic Whispering Gallery Mode Optical Sensors for Biological Applications. Laser Photonics Rev. 2020, 14 (12), 1–20. 10.1002/lpor.202000135. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
