Molecular Simulation of Surfactant Displacement of Residual Oil in Nanopores: Formation of Water Channels and Electrostatic Interaction
- PMID: 38284087
- PMCID: PMC10809248
- DOI: 10.1021/acsomega.3c09116
Molecular Simulation of Surfactant Displacement of Residual Oil in Nanopores: Formation of Water Channels and Electrostatic Interaction
Abstract
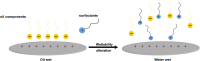
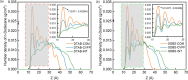
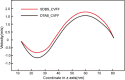
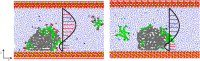
The water-oil-rock system's surfactant and electrostatic interactions are essential for removing oil droplets from rock substrates. Our work illustrates the impact of surface charge on the oil contact angle in an ideal system comprising silica, water, and dodecane; smaller contact angles are observed for more polar substrates. Modifying the polarity of the model silica surface allows for the observation of the creation of heteromolecule channels and the process of stripping crude oil while accounting for the impacts of water flow and different types of surfactant molecules. In solutions containing ionic surfactants, the injection and diffusion of water molecules between the oil layer and the silica substrate are facilitated by the disturbance of the oil molecules by the surfactant molecules. By comparing different surfactants in water flow, the characterization of water molecular channels and the stripping process of crude oil can be observed. The disruption of oil molecules by the surfactant molecules has been found to enhance the injection and diffusion of water molecules between the oil layer and the silica substrate in solutions containing ionic surfactants. The size of the contact angle and the extension of the water channel are simultaneously greatly influenced by the surfactant's molecular characteristics and the substrate's polarity. These simulation results show that several factors influence the process of water molecule channel creation that water molecules diffuse, and the detachment of oil from the silica substrate is facilitated by the migration of surfactants to the bottom of the oil molecule and the electrostatic interactions between the water molecules and the silica substrate.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- McAuliffe C. D. Oil and Gas Migration—Chemical and Physical Constraints. AAPG Bull. 1979, 63 (5), 761–781. 10.1306/2F9182CF-16CE-11D7-8645000102C1865D. - DOI
-
- Harry Dembicki J.; Anderson M. J. Secondary Migration of Oil: Experiments Supporting Efficient Movement of Separate, Buoyant Oil Phase Along Limited Conduits. AAPG Bull. 1989, 73 (8), 1018–1021. 10.1306/44B4A2DA-170A-11D7-8645000102C1865D. - DOI
-
- Dandekar A. Y.Petroleum Reservoir Rock and Fluid Properties, CRC Press: Boca Raton, 2013.
-
- Peters K. E.; Fowler M. G. Applications of Petroleum Geochemistry to Exploration and Reservoir Management. Org. Geochem. 2002, 33 (1), 5–36. 10.1016/S0146-6380(01)00125-5. - DOI
-
- Liu K.; Ostadhassan M.; Zhou J.; Gentzis T.; Rezaee R. Nanoscale Pore Structure Characterization of the Bakken Shale in the USA. Fuel 2017, 209, 567–578. 10.1016/j.fuel.2017.08.034. - DOI
LinkOut - more resources
Full Text Sources
