METformin for the MINimization of Geographic Atrophy Progression (METforMIN): A Randomized Trial
- PMID: 38284098
- PMCID: PMC10810745
- DOI: 10.1016/j.xops.2023.100440
METformin for the MINimization of Geographic Atrophy Progression (METforMIN): A Randomized Trial
Abstract
Purpose: Metformin use has been associated with a decreased risk of age-related macular degeneration (AMD) progression in observational studies. We aimed to evaluate the efficacy of oral metformin for slowing geographic atrophy (GA) progression.
Design: Parallel-group, multicenter, randomized phase II clinical trial.
Participants: Participants aged ≥ 55 years without diabetes who had GA from atrophic AMD in ≥ 1 eye.
Methods: We enrolled participants across 12 clinical centers and randomized participants in a 1:1 ratio to receive oral metformin (2000 mg daily) or observation for 18 months. Fundus autofluorescence imaging was obtained at baseline and every 6 months.
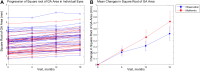
Main outcome measures: The primary efficacy endpoint was the annualized enlargement rate of the square root-transformed GA area. Secondary endpoints included best-corrected visual acuity (BCVA) and low luminance visual acuity (LLVA) at each visit.
Results: Of 66 enrolled participants, 34 (57 eyes) were randomized to the observation group and 32 (53 eyes) were randomized to the treatment group. The median follow-up duration was 13.9 and 12.6 months in the observation and metformin groups, respectively. The mean ± standard error annualized enlargement rate of square root transformed GA area was 0.35 ± 0.04 mm/year in the observation group and 0.42 ± 0.04 mm/year in the treatment group (risk difference = 0.07 mm/year, 95% confidence interval = -0.05 to 0.18 mm/year; P = 0.26). The mean ± standard error decline in BCVA was 4.8 ± 1.7 letters/year in the observation group and 3.4 ± 1.1 letters/year in the treatment group (P = 0.56). The mean ± standard error decline in LLVA was 7.3 ± 2.5 letters/year in the observation group and 0.8 ± 2.2 letters/year in the treatment group (P = 0.06). Fourteen participants in the metformin group experienced nonserious adverse events related to metformin, with gastrointestinal side effects as the most common. No serious adverse events were attributed to metformin.
Conclusions: The results of this trial as conducted do not support oral metformin having effects on reducing the progression of GA. Additional placebo-controlled trials are needed to explore the role of metformin for AMD, especially for earlier stages of the disease.
Financial disclosures: Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Age-related macular degeneration; Geographic atrophy; Metformin; Randomized controlled trial.
© 2023 by the American Academy of Ophthalmologyé.
Figures


References
-
- Wong W.L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. - PubMed
-
- Holz F.G., Strauss E.C., Schmitz-Valckenberg S., van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121:1079–1091. - PubMed
-
- Mahmoudzadeh R., Hinkle J.W., Hsu J., Garg S.J. Emerging treatments for geographic atrophy in age-related macular degeneration. Curr Opin Ophthalmol. 2021;32:294–300. - PubMed
-
- Liao D.S., Grossi F.V., El Mehdi D., et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127:186–195. - PubMed

