Reasons for failure of noninvasive prenatal test for cell-free fetal DNA in maternal peripheral blood
- PMID: 38284448
- PMCID: PMC10795076
- DOI: 10.1002/mgg3.2351
Reasons for failure of noninvasive prenatal test for cell-free fetal DNA in maternal peripheral blood
Abstract
Background: To explore reasons for the failure of noninvasive prenatal test (NIPT) for cell-free fetal DNA (cffDNA) in maternal peripheral blood, and discuss appropriate treatment schemes after the failure of the test.
Methods: Altogether 41,136 pregnant women participated in NIPT. Blood samples were taken again from pregnant women who failed the first blood collection upon their informed consent. Prenatal genetic counseling or prenatal diagnosis was recommended for pregnant women with final NIPT failure.
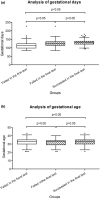
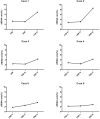
Results: The first failure rate of NIPT was 0.737% (303/41136), and the reason for the failure was the low ratio of cffDNA in 135 (44.6%) of the 303 pregnant women. After the second or third blood sampling, the final failure rate was 0.182% (75/41136). The low ratio of cffDNA was the main reason for test failure in 42 (56.0%) of the 75 pregnant women who finally failed NIPT, among whom 44 (58.7%) had underlying diseases, including 21 (47.7%) with more than two coexisting underlying diseases. Only 27 (36.0%) of the 75 pregnant women with NIPT failure underwent interventional prenatal diagnosis.
Conclusions: The main reason for NIPT failure was the low ratio of cffDNA. Postponing the gestational weeks of blood collection may improve the success rate. Resampling and retesting upon informed consent in pregnant women who failed the first test could improve the success rate. For pregnant women who finally failed NIPT, it is suggested strengthening the genetic counseling, prenatal examination, and ultrasound evaluation, and carry out interventional prenatal diagnosis if necessary.
Keywords: NIPT; cffDNA; failure.
© 2024 The Authors. Molecular Genetics & Genomic Medicine published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that the research is conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Borth, H. , Teubert, A. , Glaubitz, R. , Knippenberg, S. , Kutur, N. , Winkler, T. , & Eiben, B. (2021). Analysis of cell‐free DNA in a consecutive series of 13,607 routine cases for the detection of fetal chromosomal aneuploidies in a single center in Germany. Archives of Gynecology and Obstetrics, 303(6), 1407–1414. 10.1007/s00404-020-05856-0 - DOI - PMC - PubMed
-
- Grömminger, S. , Erkan, S. , Schöck, U. , Stangier, K. , Bonnet, J. , Schloo, R. , Schubert, A. , Prott, E. C. , Knoll, U. , Stumm, M. , von Kalle, C. , & Hofmann, W. (2015). The influence of low molecular weight heparin medication on plasma DNA in pregnant women. Prenatal Diagnosis, 35(11), 1155–1157. 10.1002/pd.4668 - DOI - PubMed
-
- Health and Family Planning Committee, P. R. China . (2016). Technical specification for prenatal screening and diagnosis of fetal free DNA in maternal peripheral blood[S].
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

