Development and validation of a combined cuproptosis and immunogenic cell death prognostic model for diffuse large B-cell lymphoma
- PMID: 38284893
- PMCID: PMC10866411
- DOI: 10.18632/aging.205399
Development and validation of a combined cuproptosis and immunogenic cell death prognostic model for diffuse large B-cell lymphoma
Abstract
Background: Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma worldwide with a high degree of heterogeneity. Cuproptosis and immunogenic cell death (ICD) have been considered to be vital for tumor progression. However, current understanding of cuproptosis and immunogenic cell death in DLBCL is still very limited. We aim to explore a prognostic model combining cuproptosis and immunogenic cell death in DLBCL.
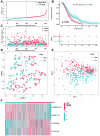
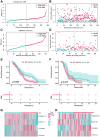
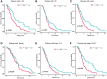
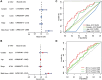
Methods: Pearson's correlation analysis was utilized to acquire lncRNAs associated with cuproptosis and immunogenic cell death. Prognostic biomarker identification and model construction involved the use of univariate Cox regression, least absolute shrinkage and selection operator (LASSO) Cox regression, and multivariate Cox regression. We assessed the predictive capability of the risk model by conducting Kaplan-Meier analysis and time-dependent ROC analysis. The analysis and comparison of immune infiltration and drug sensitivity were conducted in this study. Moreover, RT-qPCR was employed to validate the expression of lncRNAs associated with cuproptosis and immunogenic cell death in DLBCL cell lines.
Results: We identified 4 prognosis-related lncRNAs (ANKRD10-IT1, HOXB-AS1, LINC00520 and LINC01165) that were correlated with cuproptosis and immunogenic cell death. The model was verified to have a good and independent predictive ability in the prognostic prediction of DLBCL patients. Moreover, significant difference was observed in immune infiltration and drug sensitivity between high- and low-risk groups.
Conclusion: Our discoveries could enhance the comprehension of the role of cuproptosis and ICD in DLBCL, potentially offering novel viewpoints and knowledge for personalized and precise treatment of DLBCL individuals.
Keywords: DLBCL; cuproptosis; immunogenic cell death; lncRNA; prognostic model.
Conflict of interest statement
Figures



Similar articles
-
Cuproptosis-related lncRNA signature as a prognostic tool and therapeutic target in diffuse large B cell lymphoma.Sci Rep. 2024 Jun 5;14(1):12926. doi: 10.1038/s41598-024-63433-w. Sci Rep. 2024. PMID: 38839842 Free PMC article.
-
Development and validation of cuproptosis-related lncRNA signatures for prognosis prediction in colorectal cancer.BMC Med Genomics. 2023 Mar 22;16(1):58. doi: 10.1186/s12920-023-01487-x. BMC Med Genomics. 2023. PMID: 36949429 Free PMC article.
-
Development and validation of a cuproptosis-associated prognostic model for diffuse large B-cell lymphoma.Front Oncol. 2023 Jan 12;12:1020566. doi: 10.3389/fonc.2022.1020566. eCollection 2022. Front Oncol. 2023. PMID: 36713586 Free PMC article.
-
Exploring the role of long noncoding RNAs in autophagy and cuproptosis processes via immune pathways in head and neck squamous carcinoma: A systematic review of the literature.Medicine (Baltimore). 2024 Aug 23;103(34):e39335. doi: 10.1097/MD.0000000000039335. Medicine (Baltimore). 2024. PMID: 39183398 Free PMC article.
-
Cuproptosis regulation by long noncoding RNAs: Mechanistic insights and clinical implications in cancer.Arch Biochem Biophys. 2025 Mar;765:110324. doi: 10.1016/j.abb.2025.110324. Epub 2025 Feb 1. Arch Biochem Biophys. 2025. PMID: 39900259 Review.
Cited by
-
Bioinformatics-based identification of key genes for Olaparib resistance in breast cancer: prognostic implications and therapeutic relevance.Discov Oncol. 2025 Jun 18;16(1):1144. doi: 10.1007/s12672-025-02989-z. Discov Oncol. 2025. PMID: 40531367 Free PMC article.
-
Cuproptosis: A Review on Mechanisms, Role in Solid and Hematological Tumors, and Association with Viral Infections.Mediterr J Hematol Infect Dis. 2025 Jul 1;17(1):e2025052. doi: 10.4084/MJHID.2025.052. eCollection 2025. Mediterr J Hematol Infect Dis. 2025. PMID: 40636277 Free PMC article. Review.
References
-
- Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, Lawrence MS, Roemer MGM, Li AJ, Ziepert M, Staiger AM, Wala JA, Ducar MD, et al.. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018; 24:679–90. 10.1038/s41591-018-0016-8 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

