Mechanisms of telomere maintenance and associated therapeutic vulnerabilities in malignant gliomas
- PMID: 38285162
- PMCID: PMC11145458
- DOI: 10.1093/neuonc/noae016
Mechanisms of telomere maintenance and associated therapeutic vulnerabilities in malignant gliomas
Abstract
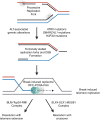
A majority of cancers (~85%) activate the enzyme telomerase to maintain telomere length over multiple rounds of cellular division. Telomerase-negative cancers activate a distinct, telomerase-independent mechanism of telomere maintenance termed alternative lengthening of telomeres (ALT). ALT uses homologous recombination to maintain telomere length and exhibits features of break-induced DNA replication. In malignant gliomas, the activation of either telomerase or ALT is nearly ubiquitous in pediatric and adult tumors, and the frequency with which these distinct telomere maintenance mechanisms (TMMs) is activated varies according to genetically defined glioma subtypes. In this review, we summarize the current state of the field of TMMs and their relevance to glioma biology and therapy. We review the genetic alterations and molecular mechanisms leading to telomerase activation or ALT induction in pediatric and adult gliomas. With this background, we review emerging evidence on strategies for targeting TMMs for glioma therapy. Finally, we comment on critical gaps and issues for moving the field forward to translate our improved understanding of glioma telomere maintenance into better therapeutic strategies for patients.
Keywords: ATRX; TERT; alternative lengthening of telomeres; gliomas; telomeres.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Z.J.R. is listed as an inventor for intellectual property related to genetic testing for TERT and other alterations in brain tumors that is managed by Duke Office of Licensing and Ventures and has been licensed to Genetron Health. D.M.A. is a Scientific Advisor Board member for Vimana Inc., MAIA Biotech Inc., Immunogenesis, and Diverse BioPharma.
Figures

Comment in
-
Telomeres in glioma: Maintenance mechanisms to therapeutic potential.Neuro Oncol. 2024 Jun 3;26(6):1025-1026. doi: 10.1093/neuonc/noae052. Neuro Oncol. 2024. PMID: 38466631 Free PMC article. No abstract available.
Similar articles
-
Telomere Maintenance Pathways in Lower-Grade Gliomas: Insights from Genetic Subtypes and Telomere Length Dynamics.Int J Mol Sci. 2025 Apr 28;26(9):4175. doi: 10.3390/ijms26094175. Int J Mol Sci. 2025. PMID: 40362411 Free PMC article.
-
Telomere elongation via alternative lengthening of telomeres (ALT) and telomerase activation in primary metastatic medulloblastoma of childhood.J Neurooncol. 2019 May;142(3):435-444. doi: 10.1007/s11060-019-03127-w. Epub 2019 Mar 4. J Neurooncol. 2019. PMID: 30830680
-
Alternative lengthening of telomeres is the major telomere maintenance mechanism in astrocytoma with isocitrate dehydrogenase 1 mutation.J Neurooncol. 2020 Mar;147(1):1-14. doi: 10.1007/s11060-020-03394-y. Epub 2020 Jan 20. J Neurooncol. 2020. PMID: 31960234 Free PMC article.
-
Telomere Length Maintenance in Cancer: At the Crossroad between Telomerase and Alternative Lengthening of Telomeres (ALT).Int J Mol Sci. 2018 Feb 18;19(2):606. doi: 10.3390/ijms19020606. Int J Mol Sci. 2018. PMID: 29463031 Free PMC article. Review.
-
Alternative mechanisms of telomere lengthening: permissive mutations, DNA repair proteins and tumorigenic progression.Mutat Res. 2013 Mar-Apr;743-744:142-150. doi: 10.1016/j.mrfmmm.2012.11.006. Epub 2012 Dec 3. Mutat Res. 2013. PMID: 23219603 Free PMC article. Review.
Cited by
-
Overcoming temozolomide resistance in glioma: recent advances and mechanistic insights.Acta Neuropathol Commun. 2025 Jun 5;13(1):126. doi: 10.1186/s40478-025-02046-4. Acta Neuropathol Commun. 2025. PMID: 40468460 Free PMC article. Review.
-
Development and Validation of a Non-Invasive Prediction Model for Glioma-Associated Epilepsy: A Comparative Analysis of Nomogram and Decision Tree.Int J Gen Med. 2025 Feb 26;18:1111-1125. doi: 10.2147/IJGM.S512814. eCollection 2025. Int J Gen Med. 2025. PMID: 40026809 Free PMC article.
-
Telomeres in glioma: Maintenance mechanisms to therapeutic potential.Neuro Oncol. 2024 Jun 3;26(6):1025-1026. doi: 10.1093/neuonc/noae052. Neuro Oncol. 2024. PMID: 38466631 Free PMC article. No abstract available.
-
Prognostic Impact of TERT Promoter Mutations in Adult-Type Diffuse Gliomas Based on WHO2021 Criteria.Cancers (Basel). 2024 May 27;16(11):2032. doi: 10.3390/cancers16112032. Cancers (Basel). 2024. PMID: 38893152 Free PMC article.
-
Telomere Maintenance and DNA Repair: A Bidirectional Relationship in Cancer Biology and Therapy.Cancers (Basel). 2025 Jul 9;17(14):2284. doi: 10.3390/cancers17142284. Cancers (Basel). 2025. PMID: 40723168 Free PMC article. Review.
References
-
- Blackburn EH, Epel ES, Lin J.. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350(6265):1193–1198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

