Emergency Department Access to Buprenorphine for Opioid Use Disorder
- PMID: 38285444
- PMCID: PMC10825722
- DOI: 10.1001/jamanetworkopen.2023.53771
Emergency Department Access to Buprenorphine for Opioid Use Disorder
Abstract
Importance: Although substantial evidence supports buprenorphine for treatment of opioid use disorder (OUD) in controlled trials, prospective study of patient outcomes in clinical implementation of emergency department (ED) buprenorphine treatment is lacking.
Objective: To examine the association between buprenorphine treatment in the ED and follow-up engagement in OUD treatment 1 month later.
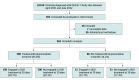
Design, setting, and participants: This multisite cohort study was conducted in 7 California EDs participating in a statewide implementation project to improve access to buprenorphine treatment. The study population included ED patients aged at least 18 years identified with OUD between April 1, 2021, and June 30, 2022. Data analysis was performed in October 2023.
Exposure: All participants were offered buprenorphine treatment for OUD (either in ED administration, prescription, or both), the uptake of which was examined as the exposure of interest.
Main outcomes and measures: The primary outcome was engagement in OUD treatment 30 days after the ED visit, determined by patient report or clinical documentation. The association of ED buprenorphine treatment with subsequent OUD treatment engagement was estimated using hierarchical generalized linear models.
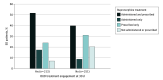
Results: This analysis included 464 ED patients with OUD. Their median age was 36.0 (IQR, 29.0-38.7) years, and most were men (343 [73.9%]). With regard to race and ethnicity, 64 patients (13.8%) self-identified as non-Hispanic Black, 183 (39.4%) as Hispanic, and 185 as non-Hispanic White (39.9%). Most patients (396 [85.3%]) had Medicaid insurance, and more than half (262 [57.8%]) had unstable housing. Self-reported fentanyl use (242 [52.2%]) and a comorbid mental health condition (328 [71.5%]) were common. Interest in buprenorphine treatment was high: 398 patients (85.8%) received buprenorphine treatment; 269 (58.0%) were administered buprenorphine in the ED and 339 (73.1%) were prescribed buprenorphine. With regard to OUD treatment engagement at 30 days after the ED visit, 198 participants (49.7%) who received ED buprenorphine treatment remained engaged compared with 15 participants (22.7%) who did not receive ED buprenorphine treatment. An association of ED buprenorphine treatment with subsequent OUD treatment engagement at 30 days was observed (adjusted risk ratio, 1.97 [95% CI, 1.27-3.07]).
Conclusions and relevance: The findings of this cohort study suggest that among patients with OUD presenting to EDs implementing low-threshold access to medications for OUD, buprenorphine treatment was associated with a substantially higher likelihood of follow-up treatment engagement 1 month later. Future research should investigate techniques to optimize both the uptake and effectiveness of buprenorphine initiation in low-threshold settings such as the ED.
Conflict of interest statement
Figures
Similar articles
-
Association Between Hospital Adoption of an Emergency Department Treatment Pathway for Opioid Use Disorder and Patient Initiation of Buprenorphine After Discharge.JAMA Health Forum. 2023 Mar 3;4(3):e230245. doi: 10.1001/jamahealthforum.2023.0245. JAMA Health Forum. 2023. PMID: 36961457 Free PMC article.
-
Implementation Facilitation to Promote Emergency Department-Initiated Buprenorphine for Opioid Use Disorder.JAMA Netw Open. 2023 Apr 3;6(4):e235439. doi: 10.1001/jamanetworkopen.2023.5439. JAMA Netw Open. 2023. PMID: 37017967 Free PMC article.
-
Emergency Department-initiated Buprenorphine and Referral to Follow-up Addiction Care: A Program Description.J Addict Med. 2022 Mar-Apr 01;16(2):216-222. doi: 10.1097/ADM.0000000000000875. J Addict Med. 2022. PMID: 34145185
-
Beyond Buprenorphine: Models of Follow-up Care for Opioid Use Disorder in the Emergeny Department.West J Emerg Med. 2020 Nov 2;21(6):257-263. doi: 10.5811/westjem.2020.7.46079. West J Emerg Med. 2020. PMID: 33207174 Free PMC article. Review.
-
Approach to buprenorphine use for opioid withdrawal treatment in the emergency setting.Am J Emerg Med. 2019 Jan;37(1):143-150. doi: 10.1016/j.ajem.2018.10.013. Epub 2018 Oct 11. Am J Emerg Med. 2019. PMID: 30355476 Review.
Cited by
-
Community-Based Medications First for Opioid Use Disorder - Care Utilization and Mortality Outcomes.Subst Abuse Rehabil. 2024 Sep 14;15:173-183. doi: 10.2147/SAR.S475807. eCollection 2024. Subst Abuse Rehabil. 2024. PMID: 39295965 Free PMC article.
-
Exploratory Analysis on the Role of Video Tools and Multidisciplinary Healthcare Providers to Aid Counselling for Buprenorphine/Naloxone Induction Within the Evaluating Microdosing in the Emergency Department Study.Cureus. 2025 May 6;17(5):e83593. doi: 10.7759/cureus.83593. eCollection 2025 May. Cureus. 2025. PMID: 40476101 Free PMC article.
-
Factors predicting access to medications for opioid use disorder for housed and unhoused patients: A machine learning approach.PLoS One. 2024 Sep 27;19(9):e0308791. doi: 10.1371/journal.pone.0308791. eCollection 2024. PLoS One. 2024. PMID: 39331614 Free PMC article.
-
Peer support for patients with opioid use disorder in the emergency department: A narrative review.J Am Coll Emerg Physicians Open. 2024 Aug 14;5(4):e13253. doi: 10.1002/emp2.13253. eCollection 2024 Aug. J Am Coll Emerg Physicians Open. 2024. PMID: 39144727 Free PMC article. Review.
-
Extended-Release 7-Day Injectable Buprenorphine for Patients With Minimal to Mild Opioid Withdrawal.JAMA Netw Open. 2024 Jul 1;7(7):e2420702. doi: 10.1001/jamanetworkopen.2024.20702. JAMA Netw Open. 2024. PMID: 38976265 Free PMC article. Clinical Trial.
References
-
- Centers for Disease Control and Prevention . National Center for Health Statistics mortality data on CDC WONDER. September 8, 2023. Accessed October 15, 2023. https://wonder.cdc.gov/mcd.html
-
- Ahmad F, Sutton P. Provisional drug overdose death counts. US Centers for Disease Control and Prevention National Center for Health Statistics. 2022. Accessed October 15, 2023. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

