Ketamine Compared With Morphine for Out-of-Hospital Analgesia for Patients With Traumatic Pain: A Randomized Clinical Trial
- PMID: 38285446
- PMCID: PMC10825723
- DOI: 10.1001/jamanetworkopen.2023.52844
Ketamine Compared With Morphine for Out-of-Hospital Analgesia for Patients With Traumatic Pain: A Randomized Clinical Trial
Abstract
Importance: Pain is a common out-of-hospital symptom among patients, and opioids are often prescribed. Research suggests that overprescribing for acute traumatic pain is still prevalent, even when limits restricting opioid prescriptions have been implemented. Ketamine hydrochloride is an alternative to opioids in adults with out-of-hospital traumatic pain.
Objective: To assess the noninferiority of intravenous ketamine compared with intravenous morphine sulfate to provide pain relief in adults with out-of-hospital traumatic pain.
Design, setting, and participants: The Intravenous Subdissociative-Dose Ketamine Versus Morphine for Prehospital Analgesia (KETAMORPH) study was a multicenter, single-blind, noninferiority randomized clinical trial comparing ketamine hydrochloride (20 mg, followed by 10 mg every 5 minutes) with morphine sulfate (2 or 3 mg every 5 minutes) in adult patients with out-of-hospital trauma and a verbal pain score equal to or greater than 5. Enrollment occurred from November 23, 2017, to November 26, 2022, in 11 French out-of-hospital emergency medical units.
Interventions: Patients were randomly assigned to ketamine (n = 128) or morphine (n = 123).
Main outcomes and measures: The primary outcome was the between-group difference in mean change in verbal rating scale pain scores measured from the time before administration of the study drug to 30 minutes later. A noninferiority margin of 1.3 was chosen.
Results: A total of 251 patients were randomized (median age, 51 [IQR, 34-69] years; 111 women [44.9%] and 140 men [55.1%] among the 247 with data available) and were included in the intention-to-treat population. The mean pain score change was -3.7 (95% CI, -4.2 to -3.2) in the ketamine group compared with -3.8 (95% CI, -4.2 to -3.4) in the morphine group. The difference in mean pain score change was 0.1 (95% CI, -0.7 to 0.9) points. There were no clinically meaningful differences for vital signs between the 2 groups. The intravenous morphine group had 19 of 113 (16.8% [95% CI, 10.4%-25.0%]) adverse effects reported (most commonly nausea [12 of 113 (10.6%)]) compared with 49 of 120 (40.8% [95% CI, 32.0%-49.6%]) in the ketamine group (most commonly emergence phenomenon [24 of 120 (20.0%)]). No adverse events required intervention.
Conclusions and relevance: In the KETAMORPH study of patients with out-of-hospital traumatic pain, the use of intravenous ketamine compared with morphine showed noninferiority for pain reduction. In the ongoing opioid crisis, ketamine administered alone is an alternative to opioids in adults with out-of-hospital traumatic pain.
Trial registration: ClinicalTrials.gov Identifier: NCT03236805.
Conflict of interest statement
Figures
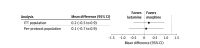
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

