Efficacy of Morning Versus Evening Latanoprost/Timolol Fixed Combination for Open-Angle Glaucoma and Ocular Hypertension: A Randomized Clinical Trial
- PMID: 38285464
- PMCID: PMC10829799
- DOI: 10.1167/tvst.13.1.21
Efficacy of Morning Versus Evening Latanoprost/Timolol Fixed Combination for Open-Angle Glaucoma and Ocular Hypertension: A Randomized Clinical Trial
Abstract
Purpose: To compare the efficacy of morning and evening latanoprost/timolol fixed-combination (LTFC) dosing in patients with primary open-angle glaucoma (POAG) and ocular hypertension.
Methods: In this double-blind, randomized clinical trial, 63 untreated Chinese patients with POAG and ocular hypertension were enrolled. All patients received LTFC and were randomized (1:1) to group 1, morning (8 AM) dosing, or group 2, evening (8 PM) dosing. Vehicle drops were used in the morning or evening, accordingly, to preserve masking. Patients were treated for 4 weeks. Outcomes included mean reduction of the 24-hour intraocular pressure (IOP) and IOP fluctuation from baseline after a 4-week treatment.
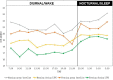
Results: Fifty-six patients were included in the final analysis. In both groups, the posttreatment IOP values were significantly lower than those at baseline at each 24-hour measuring time point. A significant difference between the groups in IOP reduction from baseline was observed at the 9:30 AM time point (4.01 ± 2.62 vs. 2.42 ± 3.23 mm Hg, evening dosing versus morning dosing group; P = 0.048). Both groups showed decreased IOP fluctuation after treatment. However, the morning dosing group had a significantly greater decrease in diurnal IOP fluctuation than that of the evening dosing group (2.04 ± 2.32 mm Hg vs. 0.50 ± 1.70 mm Hg, respectively; P = 0.012).
Conclusions: Both morning and evening LTFC dosing can effectively reduce 24-hour IOP and IOP fluctuation. Morning dosing is more likely to effectively control diurnal IOP fluctuations.
Translational relevance: This multicenter, double-blind, randomized clinical trial generates robust evidence on the optimal LTFC dosing regimen to help clinical decision-making in the treatment of raised IOP.
Conflict of interest statement
Disclosure:
Figures
References
-
- The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000; 130(4): 429–440. - PubMed
-
- Kass MA, Heuer DK, Higginbotham EJ.. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120(6): 701–713; discussion 829–830. - PubMed
-
- Garway-Heath DF, Crabb DP, Bunce C, et al. .. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015; 385(9975): 1295–1304. - PubMed
-
- Leske MC, Heijl A, Hussein M.. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003; 121(1): 48–56. - PubMed
-
- Diestelhorst M, Larsson LI.. A 12-week, randomized, double-masked, multicenter study of the fixed combination of latanoprost and timolol in the evening versus the individual components. Ophthalmology. 2006; 113(1): 70–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources