Epigenetic drift underlies epigenetic clock signals, but displays distinct responses to lifespan interventions, development, and cellular dedifferentiation
- PMID: 38285616
- PMCID: PMC10866415
- DOI: 10.18632/aging.205503
Epigenetic drift underlies epigenetic clock signals, but displays distinct responses to lifespan interventions, development, and cellular dedifferentiation
Abstract
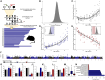
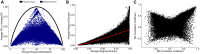
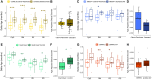
Changes in DNA methylation with age are observed across the tree of life. The stereotypical nature of these changes can be modeled to produce epigenetic clocks capable of predicting chronological age with unprecedented accuracy. Despite the predictive ability of epigenetic clocks and their utility as biomarkers in clinical applications, the underlying processes that produce clock signals are not fully resolved, which limits their interpretability. Here, we develop a computational approach to spatially resolve the within read variability or "disorder" in DNA methylation patterns and test if age-associated changes in DNA methylation disorder underlie signals comprising epigenetic clocks. We find that epigenetic clock loci are enriched in regions that both accumulate and lose disorder with age, suggesting a link between DNA methylation disorder and epigenetic clocks. We then develop epigenetic clocks that are based on regional disorder of DNA methylation patterns and compare their performance to other epigenetic clocks by investigating the influences of development, lifespan interventions, and cellular dedifferentiation. We identify common responses as well as critical differences between canonical epigenetic clocks and those based on regional disorder, demonstrating a fundamental decoupling of epigenetic aging processes. Collectively, we identify key linkages between epigenetic disorder and epigenetic clocks and demonstrate the multifaceted nature of epigenetic aging in which stochastic processes occurring at non-random loci produce predictable outcomes.
Keywords: DNA methylation; epigenetic aging; epigenetic drift; epigenetic rejuvenation; lifespan.
Conflict of interest statement
Figures


References
-
- Landau DA, Clement K, Ziller MJ, Boyle P, Fan J, Gu H, Stevenson K, Sougnez C, Wang L, Li S, Kotliar D, Zhang W, Ghandi M, et al.. Locally disordered methylation forms the basis of intratumor methylome variation in chronic lymphocytic leukemia. Cancer Cell. 2014; 26:813–25. 10.1016/j.ccell.2014.10.012 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

