A novel cysteine-rich adaptor protein is required for mucin packaging and secretory granule stability in vivo
- PMID: 38285943
- PMCID: PMC10861859
- DOI: 10.1073/pnas.2314309121
A novel cysteine-rich adaptor protein is required for mucin packaging and secretory granule stability in vivo
Abstract
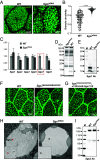
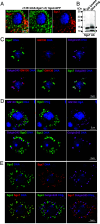
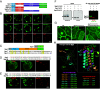
Mucins are large, highly glycosylated extracellular matrix proteins that line and protect epithelia of the respiratory, digestive, and urogenital tracts. Previous work has shown that mucins form large, interconnected polymeric networks that mediate their biological functions once secreted. However, how these large matrix molecules are compacted and packaged into much smaller secretory granules within cells prior to secretion is largely unknown. Here, we demonstrate that a small cysteine-rich adaptor protein is essential for proper packaging of a secretory mucin in vivo. This adaptor acts via cysteine bonding between itself and the cysteine-rich domain of the mucin. Loss of this adaptor protein disrupts mucin packaging in secretory granules, alters the mobile fraction within granules, and results in granules that are larger, more circular, and more fragile. Understanding the factors and mechanisms by which mucins and other highly glycosylated matrix proteins are properly packaged and secreted may provide insight into diseases characterized by aberrant mucin secretion.
Keywords: glycosylation; mucin; salivary glands; secretion; secretory granules.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Similar articles
-
Regulated Restructuring of Mucins During Secretory Granule Maturation In Vivo.Proc Natl Acad Sci U S A. 2022 Oct 25;119(43):e2209750119. doi: 10.1073/pnas.2209750119. Epub 2022 Oct 17. Proc Natl Acad Sci U S A. 2022. PMID: 36252017 Free PMC article.
-
Mucins MUC5AC and MUC5B Are Variably Packaged in the Same and in Separate Secretory Granules.Am J Respir Crit Care Med. 2022 Nov 1;206(9):1081-1095. doi: 10.1164/rccm.202202-0309OC. Am J Respir Crit Care Med. 2022. PMID: 35776514 Free PMC article.
-
Regulated mucin secretion from airway epithelial cells.Front Endocrinol (Lausanne). 2013 Sep 18;4:129. doi: 10.3389/fendo.2013.00129. Front Endocrinol (Lausanne). 2013. PMID: 24065956 Free PMC article. Review.
-
Mucin granule intraluminal organization.Am J Respir Cell Mol Biol. 2007 Feb;36(2):183-90. doi: 10.1165/rcmb.2006-0291TR. Epub 2006 Sep 7. Am J Respir Cell Mol Biol. 2007. PMID: 16960124 Free PMC article. Review.
-
Unpacking a gel-forming mucin: a view of MUC5B organization after granular release.Am J Physiol Lung Cell Mol Physiol. 2010 Jan;298(1):L15-22. doi: 10.1152/ajplung.00194.2009. Epub 2009 Sep 25. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 19783639 Free PMC article.
Cited by
-
The Role of Mucins in Cancer and Cancer Progression: A Comprehensive Review.Curr Issues Mol Biol. 2025 May 29;47(6):406. doi: 10.3390/cimb47060406. Curr Issues Mol Biol. 2025. PMID: 40699805 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

