Aspergillus and the Lung
- PMID: 38286136
- PMCID: PMC10857890
- DOI: 10.1055/s-0043-1777259
Aspergillus and the Lung
Abstract
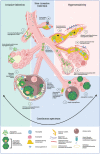
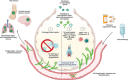
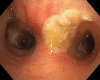
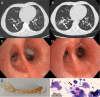
The filamentous fungus Aspergillus causes a wide spectrum of diseases in the human lung, with Aspergillus fumigatus being the most pathogenic and allergenic subspecies. The broad range of clinical syndromes that can develop from the presence of Aspergillus in the respiratory tract is determined by the interaction between host and pathogen. In this review, an oversight of the different clinical entities of pulmonary aspergillosis is given, categorized by their main pathophysiological mechanisms. The underlying immune processes are discussed, and the main clinical, radiological, biochemical, microbiological, and histopathological findings are summarized.
Thieme. All rights reserved.
Conflict of interest statement
None declared. E.V.B. is chair of the Chronic Pulmonary Aspergillosis Network (CPAnet).
Figures


References
-
- Patterson T F. Elsevier; 2019. Aspergillus Species.
-
- Kosmidis C, Denning D W. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70(03):270–277. - PubMed
-
- Brown G D, Denning D W, Gow N AR, Levitz S M, Netea M G, White T C. Hidden killers: human fungal infections. Sci Transl Med. 2012;4(165):165rv13. - PubMed
-
- Ainsworth G C. Cambridge University Press;; 1976. Introduction to the History of Mycology.

