Melanoma Derived Exosomes Amplify Radiotherapy Induced Abscopal Effect via IRF7/I-IFN Axis in Macrophages
- PMID: 38286661
- PMCID: PMC10987102
- DOI: 10.1002/advs.202304991
Melanoma Derived Exosomes Amplify Radiotherapy Induced Abscopal Effect via IRF7/I-IFN Axis in Macrophages
Abstract
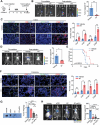
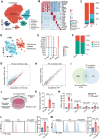
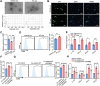
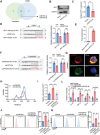
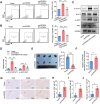
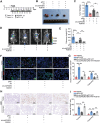
Radiotherapy (RT) can induce tumor regression outside the irradiation field, known as the abscopal effect. However, the detailed underlying mechanisms remain largely unknown. A tumor-bearing mouse model is successfully constructed by inducing both subcutaneous tumors and lung metastases. Single-cell RNA sequencing, immunofluorescence, and flow cytometry are performed to explore the regulation of tumor microenvironment (TME) by RT. A series of in vitro assays, including luciferase reporter, RNA Pulldown, and fluorescent in situ hybridization (FISH) assays, are performed to evaluate the detailed mechanism of the abscopal effect. In addition, in vivo assays are performed to investigate combination therapy strategies for enhancing the abscopal effect. The results showed that RT significantly inhibited localized tumor and lung metastasis progression and improved the TME. Mechanistically, RT promoted the release of tumor-derived exosomes carrying circPIK3R3, which is taken up by macrophages. circPIK3R3 promoted Type I interferon (I-IFN) secretion and M1 polarization via the miR-872-3p/IRF7 axis. Secreted I-IFN activated the JAK/STAT signaling pathway in CD8+ T cells, and promoted IFN-γ and GZMB secretion. Together, the study shows that tumor-derived exosomes promote I-IFN secretion via the circPIK3R3/miR-872-3p/IRF7 axis in macrophages and enhance the anti-tumor immune response of CD8+ T cells.
Keywords: IRF7; I‐IFNs; abscopal effect; melanoma; radiotherapy.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Siegel R. L., Miller K. D., Jemal A., CA Cancer J. Clin. 2018, 68, 7. - PubMed
-
- Proietti I., Skroza N., Bernardini N., Tolino E., Balduzzi V., Marchesiello A., Michelini S., Volpe S., Mambrin A., Mangino G., Romeo G., Maddalena P., Rees C., Potenza C., Cancers 2020, 12, 2801. - PubMed
-
- Mameghan H., Knittel T., Med. J. Aust. 1988, 149, 474. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
