Mixed-methods randomised study exploring the feasibility and acceptability of eye-movement desensitisation and reprocessing for improving the mental health of traumatised survivors of intensive care following hospital discharge: protocol
- PMID: 38286705
- PMCID: PMC10826543
- DOI: 10.1136/bmjopen-2023-081969
Mixed-methods randomised study exploring the feasibility and acceptability of eye-movement desensitisation and reprocessing for improving the mental health of traumatised survivors of intensive care following hospital discharge: protocol
Abstract
Introduction: Post-traumatic symptoms are common among patients discharged from intensive care units (ICUs), adversely affecting well-being, increasing healthcare utilisation and delaying return to work. Non-pharmacological approaches (eg, music, therapeutic touch and patient diaries) have been suggested as candidate interventions and trauma-focused psychological interventions have been endorsed by international bodies. Neither category of intervention is supported by definitive evidence of long-term clinical effectiveness in patients who have been critically ill. This study assesses the feasibility and acceptability of using eye-movement desensitisation and reprocessing (EMDR) to improve the mental health of ICU survivors.
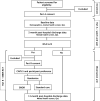
Methods and analysis: EMERALD is a multicentre, two-part consent, pilot feasibility study, recruiting discharged ICU survivors from three hospitals in the UK. We are gathering demographics and measuring post-traumatic symptoms, anxiety, depression and quality of life at baseline. Two months after discharge, participants are screened for symptoms of post-traumatic stress disorder (PTSD) using the Impact of Events Scale-Revised (IES-R). Patients with IES-R scores<22 continue in an observation arm for 12 month follow-up. IES-R scores≥22 indicate above-threshold PTSD symptoms and trigger invitation to consent for part B: a randomised controlled trial (RCT) of EMDR versus usual care, with 1:1 randomisation. The study assesses feasibility (recruitment, retention and intervention fidelity) and acceptability (through semistructured interviews), using a theoretical acceptability framework. Clinical outcomes (PTSD, anxiety, depression and quality of life) are collected at baseline, 2 and 12 months, informing power calculations for a definitive RCT, with quantitative and qualitative data convergence guiding RCT refinements.
Ethics and dissemination: This study has undergone external expert peer review and is funded by the National Institute for Health and Care Research (grant number: NIHR302160). Ethical approval has been granted by South Central-Hampshire A Research Ethics Committee (IRAS number: 317291). Results will be disseminated through the lay media, social media, peer-reviewed publication and conference presentation.
Trial registration number: NCT05591625.
Keywords: Anxiety disorders; Feasibility Studies; INTENSIVE & CRITICAL CARE; MENTAL HEALTH; PSYCHIATRY.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- The Faculty of Intensive Care Medicine . FICM position statement and provisional guidance: recovery and rehabilitation for patients following the pandemic; 2020.
-
- Johanna Josepha Op’t Hoog SA, Eskes AM, Johanna van Mersbergen-de Bruin MP, et al. . The effects of intensive care unit-initiated transitional care interventions on elements of post-intensive care syndrome: a systematic review and meta-analysis. Aust Crit Care 2022;35:309–20. 10.1016/j.aucc.2021.04.010 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical