Dorsal hippocampus to nucleus accumbens projections drive reinforcement via activation of accumbal dynorphin neurons
- PMID: 38286800
- PMCID: PMC10825206
- DOI: 10.1038/s41467-024-44836-9
Dorsal hippocampus to nucleus accumbens projections drive reinforcement via activation of accumbal dynorphin neurons
Abstract
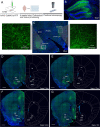
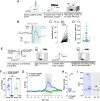
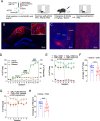
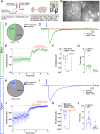
The hippocampus is pivotal in integrating emotional processing, learning, memory, and reward-related behaviors. The dorsal hippocampus (dHPC) is particularly crucial for episodic, spatial, and associative memory, and has been shown to be necessary for context- and cue-associated reward behaviors. The nucleus accumbens (NAc), a central structure in the mesolimbic reward pathway, integrates the salience of aversive and rewarding stimuli. Despite extensive research on dHPC→NAc direct projections, their sufficiency in driving reinforcement and reward-related behavior remains to be determined. Our study establishes that activating excitatory neurons in the dHPC is sufficient to induce reinforcing behaviors through its direct projections to the dorso-medial subregion of the NAc shell (dmNAcSh). Notably, dynorphin-containing neurons specifically contribute to dHPC-driven reinforcing behavior, even though both dmNAcSh dynorphin- and enkephalin-containing neurons are activated with dHPC stimulation. Our findings unveil a pathway governing reinforcement, advancing our understanding of the hippocampal circuity's role in reward-seeking behaviors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

