PRL2 regulates neutrophil extracellular trap formation which contributes to severe malaria and acute lung injury
- PMID: 38286811
- PMCID: PMC10825202
- DOI: 10.1038/s41467-024-45210-5
PRL2 regulates neutrophil extracellular trap formation which contributes to severe malaria and acute lung injury
Abstract
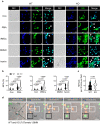
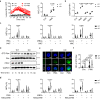
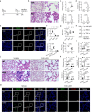
Excessive host immune responses contribute to severe malaria with high mortality. Here, we show that PRL2 in innate immune cells is highly related to experimental malaria disease progression, especially the development of murine severe malaria. In the absence of PRL2 in myeloid cells, Plasmodium berghei infection results in augmented lung injury, leading to significantly increased mortality. Intravital imaging revealed greater neutrophilic inflammation and NET formation in the lungs of PRL2 myeloid conditional knockout mice. Depletion of neutrophils prior to the onset of severe disease protected mice from NETs associated lung injury, and eliminated the difference between WT and PRL2 CKO mice. PRL2 regulates neutrophil activation and NET accumulation via the Rac-ROS pathway, thus contributing to NETs associated ALI. Hydroxychloroquine, an inhibitor of PRL2 degradation alleviates NETs associated tissue damage in vivo. Our findings suggest that PRL2 serves as an indicator of progression to severe malaria and ALI. In addition, our study indicated the importance of PRL2 in NET formation and tissue injury. It might open a promising path for adjunctive treatment of NET-associated disease.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Navigating the terrain of neutrophil extracellular traps in severe malaria pathogenesis.Trends Parasitol. 2024 Apr;40(4):278-279. doi: 10.1016/j.pt.2024.03.001. Epub 2024 Mar 13. Trends Parasitol. 2024. PMID: 38485580
-
Targeting Neutrophils to Prevent Malaria-Associated Acute Lung Injury/Acute Respiratory Distress Syndrome in Mice.PLoS Pathog. 2016 Dec 7;12(12):e1006054. doi: 10.1371/journal.ppat.1006054. eCollection 2016 Dec. PLoS Pathog. 2016. PMID: 27926944 Free PMC article.
-
Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury.Sci Rep. 2016 Nov 16;6:37252. doi: 10.1038/srep37252. Sci Rep. 2016. PMID: 27849031 Free PMC article.
-
The role of neutrophil extracellular traps in acute lung injury.Front Immunol. 2022 Jul 29;13:953195. doi: 10.3389/fimmu.2022.953195. eCollection 2022. Front Immunol. 2022. PMID: 35967320 Free PMC article. Review.
-
Progression of Cystic Fibrosis Lung Disease from Childhood to Adulthood: Neutrophils, Neutrophil Extracellular Trap (NET) Formation, and NET Degradation.Genes (Basel). 2019 Feb 26;10(3):183. doi: 10.3390/genes10030183. Genes (Basel). 2019. PMID: 30813645 Free PMC article. Review.
Cited by
-
Platelet membrane-coated nanoparticles inhibit platelet activation and neutrophil extracellular traps formation in acute lung injury.J Transl Med. 2025 Jul 25;23(1):841. doi: 10.1186/s12967-025-06649-2. J Transl Med. 2025. PMID: 40713829 Free PMC article.
-
Neutrophil Spatiotemporal Regulatory Networks: Dual Roles in Tumor Growth Regulation and Metastasis.Biomedicines. 2025 Jun 14;13(6):1473. doi: 10.3390/biomedicines13061473. Biomedicines. 2025. PMID: 40564191 Free PMC article. Review.
-
TLR9/NF-κB-mediated dendritic cell activation by neutrophil extracellular traps drives pathogenesis in experimental cerebral malaria.J Neuroinflammation. 2025 Aug 26;22(1):206. doi: 10.1186/s12974-025-03531-2. J Neuroinflammation. 2025. PMID: 40859255 Free PMC article.
-
TRIM21 knockdown alleviates hemorrhage induced hepatic ischemia reperfusion injury by suppressing ferroptosis-induced NETs.Sci Rep. 2025 May 14;15(1):16731. doi: 10.1038/s41598-025-99182-7. Sci Rep. 2025. PMID: 40369118 Free PMC article.
-
PRL2 negatively regulates FcεRI mediated activation of mast cells.Cell Death Dis. 2025 Apr 21;16(1):322. doi: 10.1038/s41419-025-07649-2. Cell Death Dis. 2025. PMID: 40258807 Free PMC article.
References
-
- World Health Organization. World malaria report 2023 (2023).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

