Desloratadine alleviates ALS-like pathology in hSOD1G93A mice via targeting 5HTR2A on activated spinal astrocytes
- PMID: 38286832
- PMCID: PMC11053015
- DOI: 10.1038/s41401-023-01223-2
Desloratadine alleviates ALS-like pathology in hSOD1G93A mice via targeting 5HTR2A on activated spinal astrocytes
Abstract
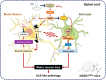
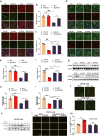
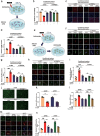
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease with progressive loss of motor neurons in the spinal cord, cerebral cortex and brain stem. ALS is characterized by gradual muscle atrophy and dyskinesia. The limited knowledge on the pathology of ALS has impeded the development of therapeutics for the disease. Previous studies have shown that autophagy and astrocyte-mediated neuroinflammation are involved in the pathogenesis of ALS, while 5HTR2A participates in the early stage of astrocyte activation, and 5HTR2A antagonism may suppress astrocyte activation. In this study, we evaluated the therapeutic effects of desloratadine (DLT), a selective 5HTR2A antagonist, in human SOD1G93A (hSOD1G93A) ALS model mice, and elucidated the underlying mechanisms. HSOD1G93A mice were administered DLT (20 mg·kg-1·d-1, i.g.) from the age of 8 weeks for 10 weeks or until death. ALS onset time and lifespan were determined using rotarod and righting reflex tests, respectively. We found that astrocyte activation accompanying with serotonin receptor 2 A (5HTR2A) upregulation in the spinal cord was tightly associated with ALS-like pathology, which was effectively attenuated by DLT administration. We showed that DLT administration significantly delayed ALS symptom onset time, prolonged lifespan and ameliorated movement disorders, gastrocnemius injury and spinal motor neuronal loss in hSOD1G93A mice. Spinal cord-specific knockdown of 5HTR2A by intrathecal injection of adeno-associated virus9 (AAV9)-si-5Htr2a also ameliorated ALS pathology in hSOD1G93A mice, and occluded the therapeutic effects of DLT administration. Furthermore, we demonstrated that DLT administration promoted autophagy to reduce mutant hSOD1 levels through 5HTR2A/cAMP/AMPK pathway, suppressed oxidative stress through 5HTR2A/cAMP/AMPK/Nrf2-HO-1/NQO-1 pathway, and inhibited astrocyte neuroinflammation through 5HTR2A/cAMP/AMPK/NF-κB/NLRP3 pathway in the spinal cord of hSOD1G93A mice. In summary, 5HTR2A antagonism shows promise as a therapeutic strategy for ALS, highlighting the potential of DLT in the treatment of the disease. DLT as a 5HTR2A antagonist effectively promoted autophagy to reduce mutant hSOD1 level through 5HTR2A/cAMP/AMPK pathway, suppressed oxidative stress through 5HTR2A/cAMP/AMPK/Nrf2-HO-1/NQO-1 pathway, and inhibited astrocytic neuroinflammation through 5HTR2A/cAMP/AMPK/NF-κB/NLRP3 pathway in the spinal cord of hSOD1G93A mice.
Keywords: NLRP3 inflammasome activation; amyotrophic lateral sclerosis; desloratadine; hSOD1G93A mice; serotonin receptor 2A; spinal astrocytes.
© 2024. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests. All institutional and national guidelines for the care and use of laboratory animals were followed.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

