Two way workable microchanneled hydrogel suture to diagnose, treat and monitor the infarcted heart
- PMID: 38286997
- PMCID: PMC10824767
- DOI: 10.1038/s41467-024-45144-y
Two way workable microchanneled hydrogel suture to diagnose, treat and monitor the infarcted heart
Abstract
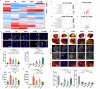
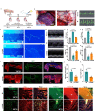
During myocardial infarction, microcirculation disturbance in the ischemic area can cause necrosis and formation of fibrotic tissue, potentially leading to malignant arrhythmia and myocardial remodeling. Here, we report a microchanneled hydrogel suture for two-way signal communication, pumping drugs on demand, and cardiac repair. After myocardial infarction, our hydrogel suture monitors abnormal electrocardiogram through the mobile device and triggers nitric oxide on demand via the hydrogel sutures' microchannels, thereby inhibiting inflammation, promoting microvascular remodeling, and improving the left ventricular ejection fraction in rats and minipigs by more than 60% and 50%, respectively. This work proposes a suture for bidirectional communication that acts as a cardio-patch to repair myocardial infarction, that remotely monitors the heart, and can deliver drugs on demand.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Injectable Shear-Thinning Hydrogels for Minimally Invasive Delivery to Infarcted Myocardium to Limit Left Ventricular Remodeling.Circ Cardiovasc Interv. 2016 Oct;9(10):e004058. doi: 10.1161/CIRCINTERVENTIONS.116.004058. Circ Cardiovasc Interv. 2016. PMID: 27729419 Free PMC article.
-
Injection of autologous bone marrow cells in hyaluronan hydrogel improves cardiac performance after infarction in pigs.Am J Physiol Heart Circ Physiol. 2014 Apr 1;306(7):H1078-86. doi: 10.1152/ajpheart.00801.2013. Epub 2014 Feb 7. Am J Physiol Heart Circ Physiol. 2014. PMID: 24508641
-
Evaluation of a polyurethane-reinforced hydrogel patch in a rat right ventricle wall replacement model.Acta Biomater. 2020 Jan 1;101:206-218. doi: 10.1016/j.actbio.2019.10.026. Epub 2019 Oct 22. Acta Biomater. 2020. PMID: 31654774 Free PMC article.
-
A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation.J Am Coll Cardiol. 2013 Jul 23;62(4):263-71. doi: 10.1016/j.jacc.2013.02.092. Epub 2013 May 15. J Am Coll Cardiol. 2013. PMID: 23684677 Review.
-
Clinical aspects of left ventricular diastolic function assessed by Doppler echocardiography following acute myocardial infarction.Dan Med Bull. 2001 Nov;48(4):199-210. Dan Med Bull. 2001. PMID: 11767125 Review.
Cited by
-
Recent Advances of Stretchable Nanomaterial-Based Hydrogels for Wearable Sensors and Electrophysiological Signals Monitoring.Nanomaterials (Basel). 2024 Aug 27;14(17):1398. doi: 10.3390/nano14171398. Nanomaterials (Basel). 2024. PMID: 39269060 Free PMC article. Review.
-
Biomaterials for reliable wearable health monitoring: Applications in skin and eye integration.Biomaterials. 2025 Mar;314:122862. doi: 10.1016/j.biomaterials.2024.122862. Epub 2024 Sep 29. Biomaterials. 2025. PMID: 39357154 Free PMC article. Review.
-
Recent Progress of Soft and Bioactive Materials in Flexible Bioelectronics.Cyborg Bionic Syst. 2025 Apr 29;6:0192. doi: 10.34133/cbsystems.0192. eCollection 2025. Cyborg Bionic Syst. 2025. PMID: 40302943 Free PMC article. Review.
-
Harnessing the potential of hydrogels for advanced therapeutic applications: current achievements and future directions.Signal Transduct Target Ther. 2024 Jul 1;9(1):166. doi: 10.1038/s41392-024-01852-x. Signal Transduct Target Ther. 2024. PMID: 38945949 Free PMC article. Review.
-
MXene-Graphene Oxide Heterostructured Films for Enhanced Metasurface Plasmonic Biosensing in Continuous Glucose Monitoring.Adv Sci (Weinh). 2025 Jan;12(4):e2410376. doi: 10.1002/advs.202410376. Epub 2024 Nov 21. Adv Sci (Weinh). 2025. PMID: 39569760 Free PMC article.
References
-
- Lee, D. S. et al. Prospective validation of the emergency heart failure mortality risk grad for acute heart failure: the acute congestive heart failure urgent care evaluation (ACUTE) study. Circulation.139, 1146–1156 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

