Increase in peg-asparaginase clearance as a predictor for inactivation in patients with acute lymphoblastic leukemia
- PMID: 38287133
- PMCID: PMC10997509
- DOI: 10.1038/s41375-024-02153-6
Increase in peg-asparaginase clearance as a predictor for inactivation in patients with acute lymphoblastic leukemia
Abstract
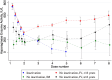
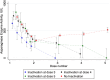
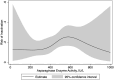
Asparaginase is an essential component of acute lymphoblastic leukemia (ALL) therapy, yet its associated toxicities often lead to treatment discontinuation, increasing the risk of relapse. Hypersensitivity reactions include clinical allergies, silent inactivation, or allergy-like responses. We hypothesized that even moderate increases in asparaginase clearance are related to later inactivation. We therefore explored mandatory monitoring of asparaginase enzyme activity (AEA) in patients with ALL aged 1-45 years treated according to the ALLTogether pilot protocol in the Nordic and Baltic countries to relate mean AEA to inactivation, to build a pharmacokinetic model to better characterize the pharmacokinetics of peg-asparaginase and assess whether an increased clearance relates to subsequent inactivation. The study analyzed 1631 real-time AEA samples from 253 patients, identifying inactivation in 18.2% of the patients. This inactivation presented as mild allergy (28.3%), severe allergy (50.0%), or silent inactivation (21.7%). A pharmacokinetic transit compartment model was used to describe AEA-time profiles, revealing that 93% of patients with inactivation exhibited prior increased clearance, whereas 86% of patients without hypersensitivity maintained stable clearance throughout asparaginase treatment. These findings enable prediction of inactivation and options for either dose increments or a shift to alternative asparaginase formulations to optimize ALL treatment strategies.
© 2024. The Author(s).
Conflict of interest statement
Jazz Pharmaceuticals has BKA serving on its advisory board and MD served as a consultant for Servier on a single occasion. All other authors state that they have no conflict of interest.
Figures

References
-
- Brigitha LJ, Fiocco M, Pieters R, Albertsen BK, Escherich G, Lopez-Lopez E, et al. Hypersensitivity to Pegylated E.coli asparaginase as first-line treatment in contemporary paediatric acute lymphoblastic leukaemia protocols: a meta-analysis of the Ponte di Legno Toxicity working group. Eur J Cancer. 2022;162:65–75. doi: 10.1016/j.ejca.2021.11.016. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

