Evaluation of the tolerability and safety of [225Ac]Ac-PSMA-I&T in patients with metastatic prostate cancer: a phase I dose escalation study
- PMID: 38287346
- PMCID: PMC10826262
- DOI: 10.1186/s12885-024-11900-y
Evaluation of the tolerability and safety of [225Ac]Ac-PSMA-I&T in patients with metastatic prostate cancer: a phase I dose escalation study
Abstract
Background: Life expectancy of patients with metastatic castration-resistant prostate cancer (mCRPC) is still limited despite several systemic treatments. Within five years after diagnosis of primary prostate cancer, 10-20% of the patients have mCRPC and curation is not an option. Radionuclide therapy (RNT) targeted against prostate-specific membrane antigen (PSMA) emerged as a new treatment option and showed effective results in patients with mCRPC. Survival benefit after [177Lu]Lu-PSMA RNT has already been demonstrated in several clinical trials. However, [225Ac]Ac-PSMA (225Ac-PSMA) appears to be an even more promising radiopharmaceutical for the treatment of mCRPC. The use of alpha emitting radionuclides offers advantages over beta emitting radionuclides due to the high linear energy transfer effective for killing tumor cells and the limited range to reduce the radiation effects on the healthy tissue. However, these results are based on retrospective data and safety data of 225Ac-PSMA are still limited. Therefore, a prospective trial is needed to determine the optimal amount of activity that can be administered.
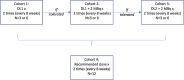
Methods: The 225Ac-PSMA-Imaging & Therapy (I&T) trial is an investigator-initiated phase I, single-center, open label, repeated dose-escalation and expansion trial. Patient with PSMA-positive mCRPC after at least one line of chemotherapy and/or one line of nonsteroidal antiandrogen will be treated with 225Ac-PSMA-I&T in increasing amount of activity per cycle. Dose-escalation following an accelerated 3 + 3 design which allows to open the next dose-level cohort in the absence of dose limiting toxicity while the previous one is still ongoing. Up to 4 treatment cohorts will be explored including up to 3 dose-escalation cohorts and one expansion cohort where patients will be administered with the recommended dose. A total of up to 30 patients will be enrolled in this trial. All patients will be evaluated for safety. Additionally, dosimetry was performed for the patients in the dose-escalation cohorts after the first 225Ac-PSMA-I&T administration.
Discussion: This trial will assess the safety and tolerability of 225Ac-PSMA-I&T in patients with mCRPC to recommend the optimal dose for the phase II trial.
Trial registration: ClinicalTrials.gov, (NCT05902247). Retrospectively registered 13 June 2023.
Keywords: Actinium-225; Clinical protocol; Dose escalation; PSMA I&T; Prostate cancer.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA.Eur Urol. 2021 Mar;79(3):343-350. doi: 10.1016/j.eururo.2020.11.013. Epub 2020 Dec 5. Eur Urol. 2021. PMID: 33293081
-
Clinical Trial Protocol for LuCAB: A Phase I-II Trial Evaluating Cabazitaxel in Combination with [177Lu]Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer.J Nucl Med. 2025 Apr 1;66(4):572-578. doi: 10.2967/jnumed.124.269252. J Nucl Med. 2025. PMID: 39978808
-
A Retrospective Analysis of the Safety and Activity of Lutetium-177-Prostate-Specific Membrane Antigen Radionuclide Treatment in Older Patients with Metastatic Castration-Resistant Prostate Cancer.Oncologist. 2020 Sep;25(9):787-792. doi: 10.1634/theoncologist.2020-0100. Epub 2020 Jun 10. Oncologist. 2020. PMID: 32430954 Free PMC article.
-
Hematologic toxicity profile and efficacy of [225Ac]Ac-PSMA-617 α-radioligand therapy of patients with extensive skeletal metastases of castration-resistant prostate cancer.Eur J Nucl Med Mol Imaging. 2022 Aug;49(10):3581-3592. doi: 10.1007/s00259-022-05778-w. Epub 2022 Apr 6. Eur J Nucl Med Mol Imaging. 2022. PMID: 35384462 Review.
-
PSMA-targeted radioligand therapy in prostate cancer: current status and future prospects.Expert Rev Anticancer Ther. 2023 Jul-Dec;23(9):959-975. doi: 10.1080/14737140.2023.2247562. Epub 2023 Aug 28. Expert Rev Anticancer Ther. 2023. PMID: 37565281 Review.
References
-
- Labriola MK, Atiq S, Hirshman N, Bitting RL. Management of men with metastatic castration-resistant prostate cancer following potent androgen receptor inhibition: a review of novel investigational therapies. Prostate Cancer Prostatic Dis. 2021;24(2):301–9. doi: 10.1038/s41391-020-00299-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

