Predictive Performance of a Gentamicin Pharmacokinetic Model in Term Neonates with Perinatal Asphyxia Undergoing Controlled Therapeutic Hypothermia
- PMID: 38287875
- PMCID: PMC11078285
- DOI: 10.1097/FTD.0000000000001166
Predictive Performance of a Gentamicin Pharmacokinetic Model in Term Neonates with Perinatal Asphyxia Undergoing Controlled Therapeutic Hypothermia
Abstract
Background: Model validation procedures are crucial when population pharmacokinetic (PK) models are used to develop dosing algorithms and to perform model-informed precision dosing. We have previously published a population PK model describing the PK of gentamicin in term neonates with perinatal asphyxia during controlled therapeutic hypothermia (TH), which showed altered gentamicin clearance during the hypothermic phase dependent on gestational age and weight. In this study, the predictive performance and generalizability of this model were assessed using an independent data set of neonates with perinatal asphyxia undergoing controlled TH.
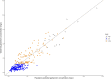
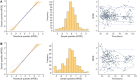
Methods: The external data set contained a subset of neonates included in the prospective observational multicenter PharmaCool Study. Predictive performance was assessed by visually inspecting observed-versus-predicted concentration plots and calculating bias and precision. In addition, simulation-based diagnostics, model refitting, and bootstrap analyses were performed.
Results: The external data set included 323 gentamicin concentrations of 39 neonates. Both the model-building and external data set included neonates from multiple centers. The original gentamicin PK model predicted the observed gentamicin concentrations with adequate accuracy and precision during all phases of controlled TH. Model appropriateness was confirmed with prediction-corrected visual predictive checks and normalized prediction distribution error analyses. Model refitting to the merged data set (n = 86 neonates with 935 samples) showed accurate estimation of PK parameters.
Conclusions: The results of this external validation study justify the generalizability of the gentamicin dosing recommendations made in the original study for neonates with perinatal asphyxia undergoing controlled TH (5 mg/kg every 36 or 24 h with gestational age 36-41 and 42 wk, respectively) and its applicability in model-informed precision dosing.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Population pharmacokinetics of vancomycin in term neonates with perinatal asphyxia treated with therapeutic hypothermia.Br J Clin Pharmacol. 2024 Jun;90(6):1418-1427. doi: 10.1111/bcp.16026. Epub 2024 Mar 7. Br J Clin Pharmacol. 2024. PMID: 38450797
-
[Association between serum cortisol levels and multiple organ dysfunction in neonates with perinatal asphyxia].Orv Hetil. 2025 Jun 15;166(24):942-952. doi: 10.1556/650.2025.33317. Print 2025 Jun 15. Orv Hetil. 2025. PMID: 40517378 Hungarian.
-
One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates.Cochrane Database Syst Rev. 2006 Jan 25;(1):CD005091. doi: 10.1002/14651858.CD005091.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2011 Nov 09;(11):CD005091. doi: 10.1002/14651858.CD005091.pub3. PMID: 16437518 Updated.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Antibiotic regimens for management of intra-amniotic infection.Cochrane Database Syst Rev. 2014 Dec 19;2014(12):CD010976. doi: 10.1002/14651858.CD010976.pub2. Cochrane Database Syst Rev. 2014. PMID: 25526426 Free PMC article.
References
-
- Douglas-Escobar M, Weiss MD. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 2015;169:397–403. - PubMed
-
- Polin RA, Committee on Fetus and Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129:1006–1015. - PubMed
-
- Musiime GM, Seale AC, Moxon SG, et al. . Risk of gentamicin toxicity in neonates treated for possible severe bacterial infection in low- and middle-income countries: systematic review. Trop Med Int Health. 2015;20:1593–1606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

