Response to experimental cold-induced pain discloses a resistant category among endurance athletes, with a distinct profile of pain-related behavior and GABAergic EEG markers: a case-control preliminary study
- PMID: 38287989
- PMCID: PMC10822956
- DOI: 10.3389/fnins.2023.1287233
Response to experimental cold-induced pain discloses a resistant category among endurance athletes, with a distinct profile of pain-related behavior and GABAergic EEG markers: a case-control preliminary study
Abstract
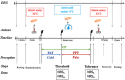
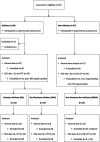
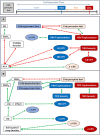
Pain is a major public health problem worldwide, with a high rate of treatment failure. Among promising non-pharmacological therapies, physical exercise is an attractive, cheap, accessible and innocuous method; beyond other health benefits. However, its highly variable therapeutic effect and incompletely understood underlying mechanisms (plausibly involving the GABAergic neurotransmission) require further research. This case-control study aimed to investigate the impact of long-lasting intensive endurance sport practice (≥7 h/week for the last 6 months at the time of the experiment) on the response to experimental cold-induced pain (as a suitable chronic pain model), assuming that highly trained individual would better resist to pain, develop advantageous pain-copying strategies and enhance their GABAergic signaling. For this purpose, clinical pain-related data, response to a cold-pressor test and high-density EEG high (Hβ) and low beta (Lβ) oscillations were documented. Among 27 athletes and 27 age-adjusted non-trained controls (right-handed males), a category of highly pain-resistant participants (mostly athletes, 48.1%) was identified, displaying lower fear of pain, compared to non-resistant non-athletes. Furthermore, they tolerated longer cold-water immersion and perceived lower maximal sensory pain. However, while having similar Hβ and Lβ powers at baseline, they exhibited a reduction between cold and pain perceptions and between pain threshold and tolerance (respectively -60% and - 6.6%; -179.5% and - 5.9%; normalized differences), in contrast to the increase noticed in non-resistant non-athletes (+21% and + 14%; +23.3% and + 13.6% respectively). Our results suggest a beneficial effect of long-lasting physical exercise on resistance to pain and pain-related behaviors, and a modification in brain GABAergic signaling. In light of the current knowledge, we propose that the GABAergic neurotransmission could display multifaceted changes to be differently interpreted, depending on the training profile and on the homeostatic setting (e.g., in pain-free versus chronic pain conditions). Despite limitations related to the sample size and to absence of direct observations under acute physical exercise, this precursory study brings into light the unique profile of resistant individuals (probably favored by training) allowing highly informative observation on physical exercise-induced analgesia and paving the way for future clinical translation. Further characterizing pain-resistant individuals would open avenues for a targeted and physiologically informed pain management.
Keywords: GABA; cold pressure test; electroencephalogram; endurance training; exercise-induced hypoalgesia; pain; pain resistance; physical exercise.
Copyright © 2024 Peier, Mouthon, De Pretto and Chabwine.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Erratum: High-Throughput Identification of Resistance to Pseudomonas syringae pv. Tomato in Tomato using Seedling Flood Assay.J Vis Exp. 2023 Oct 18;(200). doi: 10.3791/6576. J Vis Exp. 2023. PMID: 37851522
-
The type of sport matters: Pain perception of endurance athletes versus strength athletes.Eur J Pain. 2019 Apr;23(4):686-696. doi: 10.1002/ejp.1335. Epub 2018 Nov 18. Eur J Pain. 2019. PMID: 30379385
-
Beta Electroencephalographic Oscillation Is a Potential GABAergic Biomarker of Chronic Peripheral Neuropathic Pain.Front Neurosci. 2021 Feb 26;15:594536. doi: 10.3389/fnins.2021.594536. eCollection 2021. Front Neurosci. 2021. PMID: 33716642 Free PMC article.
-
American Medical Society for Sports Medicine position statement: concussion in sport.Br J Sports Med. 2013 Jan;47(1):15-26. doi: 10.1136/bjsports-2012-091941. Br J Sports Med. 2013. PMID: 23243113 Review.
-
Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation.Health Technol Assess. 2020 Sep;24(46):1-490. doi: 10.3310/hta24460. Health Technol Assess. 2020. PMID: 32975190 Free PMC article.
References
LinkOut - more resources
Full Text Sources

