Successful inguinal interstitial brachytherapy in metastatic cervical carcinoma: a case report
- PMID: 38288097
- PMCID: PMC10822930
- DOI: 10.3389/fonc.2023.1330681
Successful inguinal interstitial brachytherapy in metastatic cervical carcinoma: a case report
Abstract
Background: Treatment of metastatic cervical cancer is a tricky issue. Currently, the National Comprehensive Cancer Network (NCCN) guideline recommends chemotherapy combined with bevacizumab for recurrent or metastatic cervical cancer. Still, the recurrence rate is high and the survival rate is low after standard treatment. We urgently need to achieve a multimodal therapy approach for recurrent or metastatic cervical cancer.
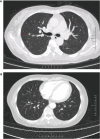
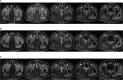
Case description: We report the case of a patient with stage IB2 cervical squamous carcinoma who developed multiple metastases within a short term after receiving first-line standard treatment, and she underwent interstitial brachytherapy after systemic therapy with an encouraging outcome. The patient developed suspected inguinal lymph node metastases after 9 months at the end of first-line therapy and multiple metastases in the inguinal lymph nodes, anterior abdominal wall, and right lung after 17 months. As the patient had residual inguinal lymph nodes after systemic therapy, she received 3D-printed template-guided interstitial brachytherapy to the inguinal lymph nodes and maintenance therapy. By Sep 2023, she had achieved a good treatment outcome with a progression-free survival (PFS) of 36 months.
Conclusion: Based on our patient response, when multiple metastases develop in the short term in early-stage cervical squamous carcinoma after first-line therapy, we may consider implementing local therapy combined with systemic therapy.
Keywords: anlotinib; case report; cervical carcinoma; interstitial brachytherapy; multiple metastases.
Copyright © 2024 Qin, Guan, Li, He, He, Tan, Deng, Liao, Wen and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- van Meir H, Kenter GG, Burggraaf J, Kroep JR, Welters MJ, Melief CJ, et al. The need for improvement of the treatment of advanced and metastatic cervical cancer, the rationale for combined chemo-immunotherapy. Anticancer Agents Med Chem (2014) 14(2):190–203. doi: 10.2174/18715206113136660372 - DOI - PubMed
-
- Cibula D, Pötter R, Planchamp F, Avall-Lundqvist E, Fischerova D, Haie Meder C, et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother Oncol (2018) 127(3):404–16. doi: 10.1016/j.radonc.2018.03.003 - DOI - PubMed
-
- cancer. Ngvc . NCCN guidelines version 1.2022 cervical cancer. Available at: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (Accessed 20 Dec 2022).
-
- Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol (2009) 27(28):4649–55. doi: 10.1200/JCO.2009.21.8909 - DOI - PMC - PubMed
-
- Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet (2017) 390(10103):1654–63. doi: 10.1016/S0140-6736(17)31607-0 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

